How to Use
Submit Request
- Download the appropriate service request form: click here.
- Complete all required fields electronically. Input your sample ID with letters, digits and/or underscores. Please do not use spaces, slashes, or any other special characters to be used for the sample name.
- Email the completed form as an attachment to [email protected].
- If a PO is needed, a quote will be created based on your service request form.
- The TCGB staff will follow up with you by email regarding your request.
**Important: TCGB will not accept samples without an emailed copy of the request form and fund account number (or PO) to [email protected]. We can only accept samples after your submission form has been confirmed via email.
Sample Preparation
miRNA/RNA/DNA Isolation
Please use the following kits in preparing your sample(s) for testing.
For miRNA isolation, use Qiagen miRNeasy Mini Kit (cat#: 217004)
For RNA/DNA isolation, use column-based purification protocol, such as Qiagen RNeasy Mini Kit (cat #: 74104) or Qiagen RNeasy Micro Kit (cat #: 74004). Please note that RNA isolated by using the Trizol method needs to be purified using Qiagen column.
For submissions of RNA or DNA, please see submission form for the minimum volume needed for requested services.
10X Single Cells for Library Construction
For scRNA-Seq Protocols
- If starting from live cells, we will check cell viability on the microscope, and count your cells. If your cell viability is >70%, we can proceed for you: obviously the higher, the better. If your cell viability is low, you may request we still proceed but we cannot guarantee the quality of the sample or library.
- Please resuspend your sorted or disassociated cells in 1xPBS+0.04% BSA solution. The ideal concentration for single cell capture is 700-1200 cells/ul, in 100ul volume. If your sample contains high percentage EDTA or surfactants, please wash the sample twice (300 rcf, 5 minutes) with 1xPBS+0.04%BSA before you make the final single cell suspension. Please bring us an extra volume of clean 1xPBS+BSA buffer with your samples in case the sample requires dilution.
- If starting from nuclei suspension, please resuspend your nuclei in 1xPBS+1% BSA solution. The ideal concentration for single nucleus capture is 700-1200 cells/ul. If your sample contains high percentage EDTA or surfactants, please wash the sample twice (500 rcf, 5minutes) with 1xPBS+1%BSA before you make the final single nucleus suspension. Please bring us an extra volume of clean 1xPBS+BSA buffer with your samples in case the sample requires dilution.
- If submitting feature barcoded samples, please note that Biolegend’s TotalSeq-A kit is not compatible to 10x platforms; please use their TotalSeq-B kit.
For scATAC-Seq Protocols
- Please resuspend your nuclei in diluted “Nuclei Buffer” at a concentration relative to the number of nuclei you intend to target (refer to Chromium Next GEM Single Cell ATAC Reagent Kits v2 User Guide). TCGB can provide an aliquot of 20x concentrated Nuclei Buffer before you prepare your nuclei suspension. This should be diluted 1 to 20 in RNase free water before nuclei resuspension. Ideal nuclei concentration is 3000-5000 nuclei/ul.
For scMultiome Protocols
- Please resuspend your nuclei in diluted “Nuclei Buffer” with DTT and RNase Inhibitorat a concentration relative to the number of nuclei you intend to target (refer to Chromium Next GEM Single Cell Multiome ATAC + Gene Expression Reagent Kits User Guide). TCGB can provide an aliquot of 20x concentrated Nuclei Buffer before you prepare your nuclei suspension. This should be diluted 1 to 20 in RNase free water before nuclei resuspension. Ideal nuclei concentration is 3000-5000 nuclei/ul.
GeoMx DSP
Please see the below for slide preparation. Please contact TPCL if assistance with slide preparation is needed.
- 4 μm-6 μm unstained sections mounted on adhesive/positively charged slides are required, e.g., Superfrost Plus; Leica X-tra-adhesive (Cat#: 3800050). For TMA, bone marrow tissue and mRNA DSP samples, Leica Bond plus slides (Cat#: S21.2113.A) are recommended.
- Dry sectioned slides at 42C with a vent for at least 4 hours to overnight. Bake at 65C for 1 hour.
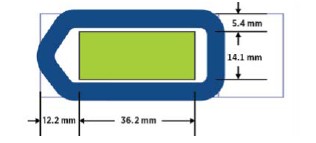
- Ideally, tissue sections should be placed in the center of the slide and be no larger than 36.2 mm wide by 14.1 mm high. If sections are larger than this size or placed off canter, it is possible that the tissue located in blue area cannot be measured.
- Tissue less than 3 years old is preferred. We recommend cutting sections fresh for best performance with RNA. Protein samples can be fresh cut or previously slide mounted.
CosMx SMI
Please see the below for slide preparation. Please contact TPCL if assistance with slide preparation is needed.
- 4 μm-6 μm unstained sections mounted on adhesive/positively charged slides are required, e.g., Superfrost Plus; Leica X-tra-adhesive (Cat#: 3800050). For TMA, bone marrow tissue and mRNA DSP samples, Leica Bond plus slides (Cat#: S21.2113.A) are recommended.
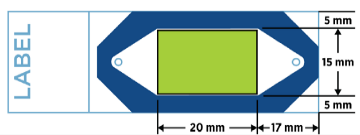
- Ideally, tissue sections should be placed in the center of the slide and be no larger than 20 mm wide by 15 mm high. If sections are larger than this size or placed off center, it is possible that the tissue located in blue area cannot be measured.
- Tissue less than 3 years old is preferred. We recommend cutting sections fresh for best performance with RNA. Protein samples can be fresh cut or previously slide mounted.
Xenium Slide
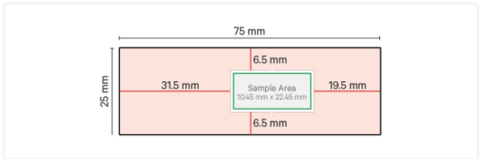
- Use the following diagram to verify that freshly placed tissue sections are compatible with the Xenium Slide. Reference the image below to draw the sample area on the back of blank slides.
- Practice correct section placement within the representative frames using non-experimental blocks
Sample Submission
- All services other than 10X Single-cell library construction: Samples can be dropped off Monday through Friday any time between 8 am to 6 pm after emailing a service request form to [email protected]
- 10x Visium (HD), Xenium, GeoMx, CosMx, and Stereoseq: To ensure timely processing and avoid delays, please schedule appointments with the following service providers at least two weeks before dropping off your samples:
- Histology: Contact TPCL for sectioning, mounting, staining, and imaging services.
- Molecular work: Contact TCGB for library preparation and sequencing services.
- 10X Single-cells library construction: Our current policy is to make appointments at least one week before you drop off your samples; we will ask you to deliver your samples on wet ice between 1pm and 4pm on Tuesday, Wednesday, or Friday.
- Please email the sample submission form to [email protected] at least one day before sample drop off.
- Please call us at (310) 206-3945 30min before bringing your samples to CHS 33-388.
- To avoid misunderstandings, please label your samples in accordance with your sample submission form and provide detailed instructions about the loading conditions of your experiments (concentration, target cells etc) if you have them.
If shipping samples, please contact the laboratory to schedule delivery. At the time of shipping, please send an email notification with a tracking number. This will allow our team to watch for your shipment. We prefer that samples be sent overnight on Monday or Tuesday to guarantee their receipt.
Please send samples to:
UCLA TCGB
CHS 38-123
650 Charles Young Drive South
Los Angeles, CA 90095
Mail Code 173517
Results and Data Retrieval
Researchers will be granted access to all hybridization images and raw data. You can request a data analysis from TCGB. You can also take advantage of additional bioinformatics tools to assist in mining, modeling, or additional analysis.
The Center has the ability to disseminate data in a variety of ways - customers can retrieve their data via Globus FTP, Amazon Web Services’ S3 storage, or Dropbox. If the data generated is too large to store on a local machine, the core can deliver data via an external hard drive for a nominal, one-time fee.