Research Areas
Main Focus of Research
I. Prostate Specific Membrane Antigen Theranostics to improve prostate cancer patient outcome.
The Theranostics Clinical Research Program is conducting clinical trials to determine the impact of PSMA PET imaging and PSMA radionuclide therapy on care and outcome of patients with prostate cancer.
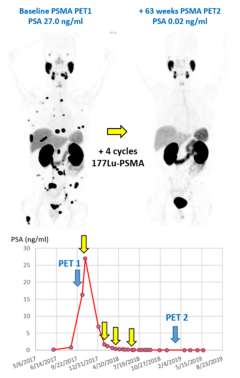
Larger image > Figure 1 : Example of a patient with metastatic castrate resistant prostate cancer responding to Lu177-PSMA radionuclide therapy.
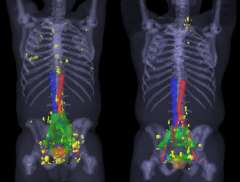
Larger image > Figure 2 : This figure shows in yellow multiple prostate cancer lesions detected by PSMA PET/CT that are outside of the standard radiation fields (green and orange fields) that would therefore fail.
II. Fibroblast activated protein as new target for nuclear theranostics.
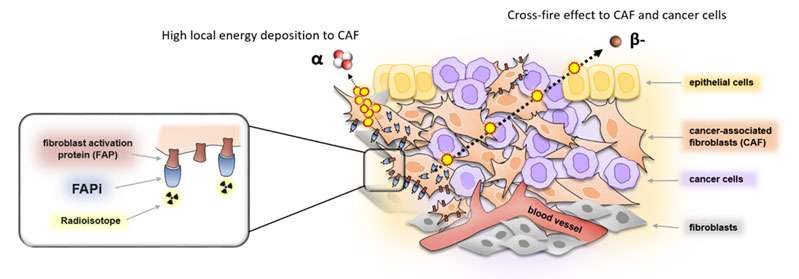
Dr Jeremie Calais has designed and initiated at UCLA exploratory clinical studies of FAPI PET targeting the tumor stroma (fibroblast activation protein) in multiple cancers. FAP is a new promising target for nuclear Theranostics.
Figure 4: FAP-target for nuclear Theranostics
Academic Research
FDA New Drug Application NDA #212642 for PSMA-11 Gallium-68
- Role: Co-Investigator and clinical study report author
- UCLA and UCSF joint application (Principal Investigators: Johannes Czernin and Thomas A Hope)
- Indication: PSMA-11 Ga 68 Injection for PET Imaging of Detection and Localization of Prostate Cancer
FDA New Drug Application NDA #130342 for Fluor-18-Choline
- Role: Co-Investigator and Blinded Independent Central Reader (BICR)
- UCSF and UCLA joint application (Principal Investigator: Thomas A Hope)
- Indication: Fluor-18 Choline for PET Imaging of Detection and Localization of parathyroid hyperactivity
Prospective Clinical Trial:
- 68Ga-PSMA-11 PET/CT for the Diagnosis of Bone Metastases in Patients With Prostate Cancer and Biochemical Progression During Androgen Deprivation Therapy
- ClinicalTrials.gov Identifier: NCT04928820
- Start date: June, 2021
- 99mTc-PSMA-I&S Biodistribution in Patients With Prostate Cancer
- ClinicalTrials.gov Identifier: NCT04857502
- Start date: June, 2021
- Randomized Trial of PSMA PET Scan Before Definitive Radiation Therapy for Prostate Cancer (PSMA DRT)
- ClinicalTrials.gov Identifier: NCT04457245
- Start Date: Sept 2020
- 68Ga-FAPi-46 PET/CT Scan in Imaging Patients With Sarcoma
- ClinicalTrials.gov Identifier: NCT04457258
- Start Date: Sept 2020
- Prospective Exploratory Study of FAPi PET/CT With Histopathology Validation in Patients With Various Cancers (FAPI PET RDRC)
- ClinicalTrials.gov Identifier: NCT04459273
- Start Date: Sept 2020
- Prospective Exploratory Study of FAPi PET/CT in Prostate Cancer With Histopathology Validation (FAPI PET Prost)
- ClinicalTrials.gov Identifier: NCT04457232
- Start Date: Sept 2020
- PET biodistribution study of 68Ga-PSMA-11 and 68Ga-FAPI-46 in patients with non-prostate cancers: an exploratory study with histopathology validation
- ClinicalTrials.gov Identifier: NCT04147494
- Start Date: Oct 2019
- Expanded Access Protocol of 68Ga-PSMA-11 for Prostate Cancer PET Imaging
- Protocol No.: NCT04348682
- Start Date: Mar 2020
- Effect of Androgen Receptor Signaling Inhibitors on 68Ga-PSMA-11 PET/CT Imaging in Patients With Metastatic Castration-Resistant Prostate Cancer
- ClinicalTrials.gov Identifier: NCT04279561
- Start Date: Mar 2020
- Evaluation by 68Ga-PSMA-11 PET imaging of Monosodium Glutamate as a potential agent for Salivary Gland Protection under PSMA-targeted alpha-therapy: a randomized pilot imaging research study
- Protocol Number: NCT04282824
- Start Date: Jan 2020
- Randomized phase 3 Trial of 68Ga-PSMA-11 PET/CT Molecular Imaging for Prostate Cancer Salvage Radiotherapy Planning [PSMA-SRT]
- ClinicalTrials.gov Identifier: NCT03582774
- Start date: July 12, 2018
- 99mTc-SestaMIBI SPECT/CT Imaging for the Characterization of Renal Masses: Impact on Clinical Decision Making
- ClinicalTrials.gov Identifier: NCT03996850
- Start Date: Dec 7, 2018
- Impact of 68Ga-PSMA-11 PET/CT on initial and subsequent treatment strategies of patients with prostate cancer.
- ClinicalTrials.gov Identifier: NCT04050215
- Start Date: Apr 7, 2018
- PSMA-directed endoRadiothErapy of Castration-reSISTant Prostate Cancer (RESIST-PC).
A Phase II Clinical Trial- ClinicalTrials.gov Identifier: NCT03042312
- Start Date: July 12, 2017
- Prospective Single Center Trial to Compare 68Ga-PSMA-11 and Axumin PET/CT (18F-Fluciclovine) for Restaging Prostate Cancer Patients With Biochemical Recurrence After Radical Prostatectomy
- ClinicalTrials.gov Identifier: NCT03515577
- Start date: March 2, 2018
- 68Ga-PSMA PET/CT for Detection of Recurrent Prostate Cancer After Initial Therapy
- ClinicalTrials.gov Identifier: NCT02940262
- Start date: September 5, 2016
- 68Ga-PSMA-11 PET/CT for Staging of Intermediate and High Risk Prostate Cancer Prior to Radical Prostatectomy
- ClinicalTrials.gov Identifier: NCT03368547
- Start Date: December 12, 2016
Biopharma Industry Sponsored Research
- SPLASH: Study Evaluating mCRPC Treatment Using PSMA [Lu-177]-PNT2002 Therapy After Second-line Hormonal Treatment
- ClinicalTrials.gov Identifier: NCT04647526
- Sponsor: POINT biopharma.
- Start Date: Oct , 2021
- 177Lu-PSMA-617 Managed Access Program for mCRPC Patients
- ClinicalTrials.gov Identifier: NCT04825652
- Sponsor: Novartis.
- Start Date: July 2021
- ARROW: Study of I-131-1095 Radiotherapy in Combination With Enzalutamide in Patients With Metastatic Castration-resistant Prostate Cancer Who Are Chemotherapy Naive and Have Progressed on Abiraterone
- ClinicalTrials.gov Identifier: NCT03939689
- Sponsor: Progenics Pharmaceuticals, Inc.
- Start Date: Dec , 2020
- ZIRCON: 89Zr-TLX250 for PET/CT Imaging of ccRCC- ZIRCON Study (89ZR-TLX250)
- ClinicalTrials.gov Identifier: NCT03849118
- Sponsor: Telix International Pty Ltd.
- Start Date: Dec, 2020
- VISION: An International, Prospective, Open Label, Multicenter, Randomized Phase 3 Study of 177Lu-PSMA-617 in the Treatment of Patients With Progressive PSMA-positive Metastatic Castration-resistant Prostate Cancer (mCRPC)
- ClinicalTrials.gov Identifier: NCT03511664
- Sponsor: Endocyte Inc.
- Start Date: Dec 7, 2018
- AMG160: A Phase 1 Study Evaluating the Safety, Tolerability, Pharmacokinetics, and Efficacy of Prostate Specific Membrane Antigen Half-life Extended Bispecific T-cell Engager AMG 160 in Subjects With Metastatic Castration-resistant Prostate Cancer
- ClinicalTrials.gov Identifier: NCT03792841
- Sponsor: Amgen Inc.
- Start Date: Dec 7, 2018
- A Multicentre, Randomised, Dose-confirmation, Factorial Phase II Study to Evaluate the Optimal Dose of 68Ga-OPS202 as a PET Imaging Agent in Subjects With Gastroenteropancreatic Neuroendocrine Tumour (GEP-NET)
- ClinicalTrials.gov Identifier: NCT03220217
- Sponsor: Ipsen Inc.
- Start Date: Sep 20, 2017