Kidney Cancer Treatment
Find your care
We combine expertise and advanced technology to deliver the best possible outcomes. For more information, connect with a cancer care specialist at 310-794-7700.
The UCLA Kidney Cancer Program is a comprehensive team (medical, surgical, radiation) that are world-renowned experts in the diagnosis, management and treatment of the broad spectrum of kidney cancer - from localized tumors to the most complicated metastatic, or advanced, kidney cancers. There are numerous factors that are considered in assessing which kidney cancer treatment option is best suited for a patient, such as medical history, current health condition, clinical and diagnostic test results and patient preference. Other factors that are taken into account are the tumor size, location and stage of the disease. By having access to world leaders in pathology, cancer genomics, and molecular imaging, our clinicians can further optimize clinical care. Based on the final assessment, the multidisciplinary kidney cancer team will recommend atreatment plan personalized to one’s clinical situation which may include active surveillance, surgery, tumor ablation, radiation, systemic therapy, or various combinations of these options. Our team feels strongly that when various acceptable options exist, both the patient and physician must contribute to the medical decision-making process (shared decision-making).
UCLA’s Kidney Cancer Program offers various treatment options for kidney cancer including:
- Active Surveillance
- Surgical Treatments (open and minimally invasive / robotic surgery)
- Tumor Ablation
- Conventional and Stereotactic Radiation
- Surgical Resection of Distant Disease (Metastasectomy)
- Immunotherapy (IL-2 and Checkpoint Inhibitors)
- Targeted Therapy
- Clinical Trials (Novel / Experimental Therapy)
Genetic Risk Assessment Program
The UCLA Genitourinary Cancer Genetic Risk Assessment Program focuses on investigation into the potential genetic causes of an individual’s urologic cancer. Up to 5-10% of cancers are related to a genetic predisposition. If you have been diagnosed with a urologic cancer, UCLA’s team has specific referral criteria to determine if you should pursue genetic risk assessment to evaluate for a genetic cause of cancer. For those at greatest risk, often this knowledge can more precisely tailor a treatment plan that is optimal for you.
Active Surveillance for Small Kidney (Renal) Masses
Many small renal masses are incidentally discovered and are benign or indolent (non-aggressive). Most small tumors grow very slowly and have a low potential to spread to other organs (metastasize). As all treatment options pose some risk, some patients are better served with surveillance in delayed intervention IF NECESSARY. The UCLA Kidney Cancer Program tries to individualize care to avoid unnecessary and overtreatment when feasible based on tumor and patient characteristics.
Surgical Treatment
Surgery is the most common treatment for kidney cancer, and very often, the only treatment required for tumors that have not moved outside the kidney. As Medicine is an art and there are often many approaches to your care, the UCLA Kidney Cancer Program prioritizes the approach based on the following principles:

There are important surgical decisions such as 1) does the patient need treatment? 2) can the kidney be saved? 3) should we offer a traditional open or minimally-invasive option (laparoscopy or robotics). Based on the above priorities factoring tumor characteristics and patient anatomy/comorbidities, the UCLA Kidney Cancer Program will offer an individualized treatment plan.
Partial Nephrectomy (Kidney-Sparing Surgery)
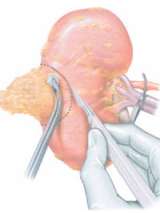
A partial nephrectomy is the surgical removal of tumor with sparing the adjacent normal kidney. While once reserved for select situations, in the 1990’s the UCLA Kidney Cancer Program expanded indications by pioneering the concept of the “elective” partial nephrectomy long before it came to be considered the standard of care. Often preservation of kidney function may improve quality and quantity of life.
Radical Nephrectomy (Complete Kidney Removal)
For large, invasive tumors, often the entire kidney needs to be removed. A radical nephrectomy can be performed in both the open and laparoscopic / robotic manner. While many centers do not perform a lymph node dissection, UCLA Kidney Cancer Program’s surgeons perform lymph node dissections whenever there is high suspicion for lymph node involvement to limit local/regional recurrences by removing any potential sites of disease.
Surgical Approach: Open, Laparoscopic, and Robotic Procedures
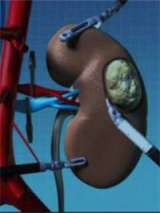
Open surgery is performed by making an incision in the patient's side, abdomen or lower back to approach the kidney. The surgical team uses their hands to perform the procedure. Laparoscopy is a minimally invasive surgical procedure, where the surgeon inserts instruments and a camera through keyhole incisions to perform the operation. This can be performed with traditional or robotic-controlled instrument (Da Vinci Robot). When needed over traditional laparoscopy, the robotic approach may provide very precise movements and the 3D camera offers an improved visual field. At UCLA the majority of operations are performed via a minimally invasive approach, however the team is adept at all surgical options to provide our patients the best outcome.
Tumor Ablation
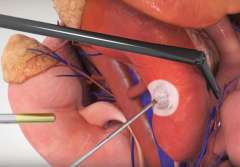
For some small tumors complete removal might not be necessary. Using extreme heat or cold can destroy/ablate a renal tumor with moderate success. The UCLA Kidney Cancer Program offers cryoablation and radiofrequency ablation to select patients. While many centers perform these procedures under general anesthesia and in the operating room, our team often performs ablation in the outpatient setting, percutaneously (through the skin), and under minimal sedation.
Conventional and Stereotactic Radiation
For tumors that have spread outside the kidney, they may cause local symptoms. Our Radiation Oncologists offer conventional (3D conformal and Intensity-modulated radiation therapy- IMRT) and stereotactic radiation. These modalities can control distant sites of disease in the brain and bone to improve quality and quantity of life. For select high-risk patients that have no other treatment options, the team is also offering stereotactic body radiation treatment (SBRT) to the kidney.
Surgical Resection of Distant Disease (Metastasectomy)
When kidney cancer spreads outside the kidney, systemic therapy is generally the only treatment option employed by many centers. However, select patients can have sites of disease surgically removed to place them in remission or palliate symptoms. As UCLA is a world-class surgical center, the Kidney Cancer Program works closely with our liver, pancreas, neurologic, colorectal, orthopedic, thoracic, and cardiac surgeons to offer surgery when indicated.
Immunotherapy
Immunotherapy is designed to stimulate the body's own immune system to attack cancer in the setting of regional/advanced kidney cancer. Kidney cancer is very responsive to immunotherapy. IL-2 was the first approved agent for metastatic kidney cancer and UCLA did much of the early work showing some patients can be cured even with advanced disease. While most centers have stopped giving IL-2 due to cost and toxicity, as our team has been involved in IL-2 for 25 years, our program still offers it to select patients. Exciting new immunotherapy agents restore the immune systems activity to attack cancer. Agents such as Nivolimab (PD-1) and Ipilimumab (CTLA-4) are offered in the outpatient setting and may also offer patients a chance for complete disease remission.
Targeted Therapy
By understanding the genetic make up and signaling pathways for kidney cancer cells, clinicians can “put on the breaks” for tumor growth. Since 2005, nine agents have been approved that are known to stop the development/recruitment of new blood vessels (angiogenesis) and thus prohibit tumor progression. Many of these agents are oral and can be safely given and allow patients to maintain an excellent quality of life.
There are numerous advantages to these newer therapies. They can often be easily administered orally in the form of a pill. They are generally well tolerated and produce fewer side effects than traditional therapies. Although these therapies have only been FDA approved for the treatment of metastatic kidney cancer; there are several clinical trials investigating their use as a treatment for patients who are identified as being at a higher risk of developing recurrent kidney disease. We are also investigating their use in combination with immunotherapy.
For kidney cancer patients in whom surgery alone or in combination with one of the established medication regimens is not curative, enrolling in a clinical trial can offer access to cutting-edge treatments even before they are released into widespread practice.
Future Treatments and Clinical Trials
While there have been advances in the treatment of kidney cancer, nearly 14,000 individuals die each year from this disease. The UCLA Kidney Cancer Program participates in novel clinical and basic research to find new treatment options. Two principles guide our research program 1) every patient is an opportunity to learn and help others and 2) clinical trials may offer tomorrow’s treatments (cures) today. We offer research protocols to every patient ranging from tissue/blood collection during routine care to learn about the disease to systemic (medical) therapy clinical trials when traditional medication may not be sufficient.
Many of our trials offer patients a more aggressive treatment approach to combat their disease. The majority of our protocols offer patients an existing standard medication plus an additional novel treatment option with the goal that this improves outcomes. Our team has participated in various trials that have led to FDA approval, providing our patients with medications years before available in the community.
If you are interested in finding out more about these protocols, ask a member of the UCLA Kidney Cancer Program team about current trials being conducted at UCLA.