Gynecologic Cancers
Find your care
Radiation oncologists use a patient-centered approach and individualized treatment plans. To learn more and make an appointment with a UCLA radiation oncologist, contact us at:
Westwood - 310-825-9775
Santa Clarita - 661-287-0010
Santa Monica - 424-259-8777
Radiation Therapy for Gynecologic Cancers
UCLA Multidisciplinary Expert Care
Our Mission is U
At UCLA we have a strong and diverse team of physicians dedicated to the care and management of women with gynecologic malignancies. We meet weekly to discuss cases as a group in order to provide an integrated and comprehensive treatment plan for patients. We provide radiation services both at the UCLA Westwood and Santa Monica campus. The radiation oncology service provides comprehensive treatment options.
External Beam Simulation and Treatment Process
Planning Session (simulation)
Do I need to do anything to prepare for the simulation
There are different things that need to be done prior to simulation depending on the reason and type of radiation you will be receiving (external beam radiation vs brachytherapy). Your doctor will review specific instructions with you at the time of your consultation but below are some general instructions for common radiation indications:
External beam radiation after surgery for endometrial or cervical cancer
About an hour prior to your simulation we will have you come to the clinic and the physician will place 3 small gold marker seeds near the top of the vagina (vaginal apex). The purpose of doing this is so that the physician can easily identify the vaginal apex on your CT scan. This is important because it helps us determine "how high" and "how low" to treat you.
This marker seed placement is done in one of the clinic rooms, takes about 5 minutes, and is very easy. No sedation or pain medications are needed. You will be put into the same position as you would be for a regular pelvic examination. The doctor will gently place a speculum inside the vagina and will then place the marker seeds. You may feel a brief pinch when the seed is placed. Significant bleeding is not expected or anticipated during this.
The marker seeds are left in permanently. It is safe for these to remain in the body and it is not expected that you will experience any reactions in the short or long term because of these marker seeds. These seeds will not cause you to set off alarms when going through the airport.
After the marker seeds have been placed we ask that you drink 30 ounces of water starting about 30 minutes prior to your simulation. In addition, it is best if you try to evacuate your bowels prior to coming to the clinic.
What happens during simulation?
The purpose of the simulation is to perform a CT scan of the area that we are going to treat in a position that we can reproduce for daily. The most typical treatment position is having the patient laying down flat on their back with their arms up on their chest. The CT scan from this simulation is sent to our dosimetrists and physicists and is used to plan how your radiation will be delivered based on your specific anatomy. The position that you are simulated in is very important because all of the radiation treatment planning is based on this position. Since the room that you are simulated in is different from the room where your radiation treatments occur we need to make sure that we can set you up the same way in both rooms. So in essence we are "simulating" what's going to happen for your actual radiation treatments.
The type of simulation that is performed will depend on whether you are going to receive 3D-conformal radiation to the pelvis or intensity modulated radiation therapy with daily image guidance (IMRT/IGRT).
3D conformal radiation to the pelvis
For this type of simulation the patient is asked to come in with a comfortably full bladder. She will lie down on the CT scanner with her arms, most typically, on her chest. A special cradle will be molded around the legs below the knees to keep them in place. Once the patient is in position a CT scan will be performed of the pelvic region. No oral or IV contrast is used for this simulation and you do not need to fast prior to this simulation. At the end of the simulation a few small tattoos will be placed to help with alignment when you return to start your actual treatments. The entire simulation takes about 1 hour.
IMRT/IGRT
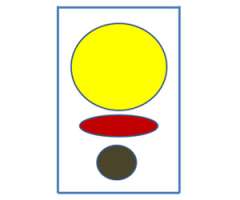
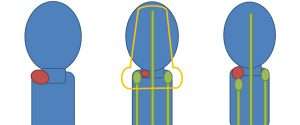
The simulation process when a patient is going to receive IMRT/IGRT takes an extra step (a bladder empty scan is done in addition to a bladder full one). The reason for this can be seen and explained through the following cartoons:
In these cartoons the yellow circle represents the bladder, the red oval represents the vagina, the brown circle represents the rectum, and the blue outline represents the area that will be treated with radiation. The first figure represents a typical example of the radiation field for 3D-conformal radiation. A large enough radiation field is used so we can ensure that all the targets are included with a generous margin. In treating with a larger margin organ motion (ie bladder/rectum being full vs empty) aren’t as relevant because the target will always fall within the radiation field.
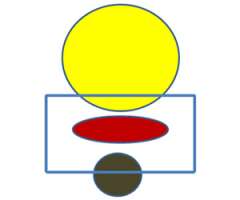
When using IMRT we are able to sculpt the radiation dose to the areas at risk for cancer while limiting the dose to normal tissues that don’t need to be treated.
So in this cartoon one can see that the target for radiation is really the upper vagina (red oval) and tissues around it and the radiation field in blue is just covering this area with a small margin extending into the bladder and rectum.
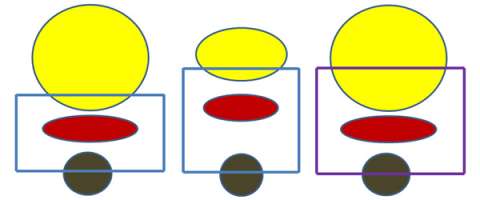
Because we are using smaller margins it is imperative that we understand how organ motion (ie rectal/bladder filling) moves the target so that we can ensure we are properly treating the intended target. To accomplish this we do the simulation with an empty rectum (ie making sure the patient has a bowel movement prior to simulation and is not constipated) and do a simulation with the bladder full and another one with the bladder empty. What this allows us to do is to see how the target moves based on your bladder filling. The target is contoured on both the bladder empty and the bladder full scans and is then fused together. This fused target volume gives us confidence that the target will fall within this volume on a daily basis.
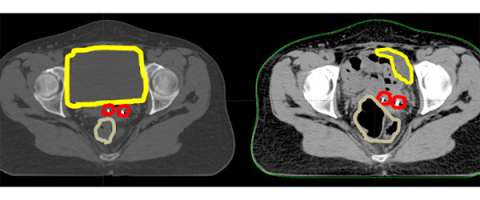
In order to be 100% sure that the target is being appropriately treated on a daily basis an image is obtained prior to your treatment daily and is reviewed to ensure that the target is indeed within the treatment volume. This is what is referred to as the image guidance portion of the treatment.
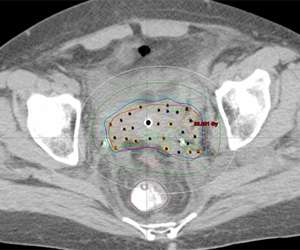
Here is an axial CT scan slice of an actual patient with her bladder full on the left and her bladder empty on the right. You can see that with the bladder (yellow) empty that additional bowel falls within the pelvis and that the marker seeds (red) are in a different place (also in part due to increased air in the rectum (brown). This example demonstrates the importance of daily image guidance and making sure that your bladder is consistently full during the course of your treatment.
What happens after the simulation is done?
We will start working on your plan and patients typically start treatment within 1 week from the time of their simulation. Prior to starting your treatment a port film is done. Sometimes this is done on the same day that you will be starting treatment and other times it's done the day before. The purpose of the port film is to make sure that we are setting you up in the treatment room in the same position that you were in for the simulation.
Is there anything that I need to do prior to each treatment?
Prior to each treatment you should have a comfortably full bladder and to the best of your ability try to stay regular with your bowel movements so that you don't get constipated or have too much gas.
What happens when I come in for the actual treatments?
When you come in for your simulation before you leave you will be given a schedule with your treatment start date and time. For external beam radiation we start treatments at 7 AM and complete treatment at 6 PM.
For the actual external beam radiation treatments you will be brought to the treatment room that has a linear accelerator. This is the machine that actual generates and delivers the radiation. You will be put onto the radiation treatment table in the exact same position that you were in for your simulation. Radiation therapists are involved in helping to get you set up on the table. After you are set up the therapist will step out of the room. There is a camera and microphone in the room should you need to communicate with the therapist. In all cases weekly imaging will be performed to make sure that you are setting up properly and these films are reviewed by your physician. In some cases daily imaging may be performed prior to your treatment. These films give us information about your set up but don’t really give us information about whether you are or are not responding to treatment.
After your position has been verified the treatment will begin. You will see and hear the linear accelerator moving around you. You will be laying still. You will not see anything coming out of the machine or feel anything while you are being treated. The treatment takes about 5-10 minutes to complete. After the treatment is done you will be helped off of the table and you will be ready to leave the clinic. For your daily treatments you will not typically see your physician as the treatment set-up and delivery of the radiation is done by the therapists who are specially trained to do this.
How often do I get to see my doctor while I'm on treatment?
For external beam you will see your doctor at least one time per week to discuss how you are doing on treatment. If you need to see him/her more often than you can let one of the therapists or nurses know and they can help coordinate this.
If I am going to get chemotherapy with radiation how is this coordinated?
The most common chemotherapy agent given at the same time with radiation for gynecologic malignancies is cisplatin. This medication is given intravenously once per week by a medical oncologist. On the day that you get chemotherapy you should get your chemotherapy first and then come in for your radiation treatment afterwards.
Brachytherapy Simulation and Treatment Process
Brachytherapy at UCLA - Gynecologic Cancers
Brachytherapy (brake-ee-therapy) is the oldest type of radiation treatment and was used well before the development of modern day linear accelerators that are used for external beam radiation therapy. It is used in the management of almost all cancers but most commonly in the treatment of gynecologic, breast, and prostate cancers.
The greatest advantage of brachytherapy is that the treatment is from "inside out" as opposed to "outside in". This means that instead of radiation traveling through normal tissues that don’t need to be exposed in order to reach a target located in the body brachytherapy allows a small radiation source to be brought directly to and/or near the target. This allows the greatest amount of radiation dose to be concentrated where it is needed most and the intensity of the radiation falls off very quickly thereby minimizing unnecessary radiation dose to your normal tissues.
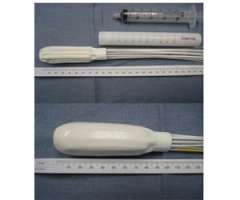
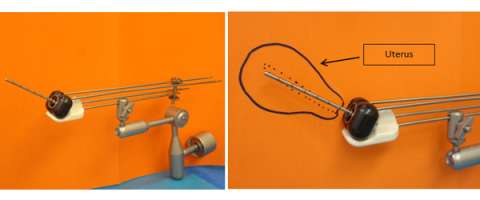
Here at UCLA we have extensive experience in brachytherapy and in particular High Dose Rate (HDR) brachytherapy. With HDR brachytherapy a single tiny (4.5 mm diameter) radioactive source of Iridium-192 is laser welded to the end of a thin, flexible stainless steel cable and is housed in a device called an afterloader. This computer guided afterloader directs the source into the treatment catheters or applicator that has been placed in the patient by the brachytherapy physicians. The source travels through each catheter in 5 mm steps, called "dwell" positions. The distribution of the radiation dose is determined by the dwell positions the source stops at and the length of time it dwells there. This ability to vary the dwell times is like having an unlimited choice of source strengths. This level of control is possible only with HDR. After the HDR treatment the source retracts into the afterloader. The patient is no longer radioactive. Finally, because the afterloader controls the radiation source, radiation exposure to the physicians, hospital staff and family members is eliminated.

On the left is an example of what a remote afterloader looks like. The middle picture shows what the inside of the afterloader looks like with the cover off. The image on the right shows how a small radiation source is welded onto the end of a cable. The motor in the afterloader pushes and pulls this cable in and out of the treatment channels.
Recently we’ve acquired the most technologically advanced HDR remote afterloader called the Flexitron. We are currently one of only a few centers in the United States to have this afterloader.
Brachytherapy is an essential component of treatment in the management of gynecologic cancers. Recent studies demonstrate that women actually live longer when they receive brachytherapy (definitive cervical cancer, medically inoperable endometrial cancer, vaginal cancer) than if brachytherapy is replaced with just external beam radiation therapy.
Not all centers have the expertise to perform brachytherapy and those that do aren’t always able to offer all types of brachytherapy (interstitial and intracavitary). There are also important advances in brachytherapy including the movement away from 2-dimensional planning to 3-dimensional planning with either CT or MRI. This is a major advance in gynecologic brachytherapy that is improving local control outcomes and reducing the risk of short and late term side effects.
It is important to be evaluated and treated at a center that has experience and expertise in offering the full range of possible treatments so that the most appropriate one is selected and performed safely.
Image Guided Brachytherapy (IGBT)
What is Image-guided Brachytherapy?
Image–guided brachytherapy (IGBT) is the use of advanced imaging techniques to make brachytherapy more accurate, safe, and effective. Brachytherapy consists of four phases:
- Placement of hollow catheters or hollow carriers
- CT or MRI imaging of the site,
- computer calculations of the dose distribution called “dosimetry” and
- robotic radiation treatment delivery with a tiny radiation source.
The imaging techniques used for interventional radiology may be used to guide the placement of the brachytherapy catheters. We currently are using these techniques in the Division of Brachytherapy at UCLA for our brachytherapy patients.
The advantages of IGBT are:
- More accurate treatment distribution throughout the targeted area
- Reduced risk of injury to healthy tissues
- The ability to treat a wide variety of cancer lesions such as those that are:
- Deep in the body
- Adjacent to blood vessels
- Too large for other treatment techniques
Image guided brachytherapy is a significant advance in the treatment of gynecologic malignancies but is highly technique dependent and is done best by centers that have a high volume of cases. Prior to image guided brachytherapy 2D images were taken with brachytherapy applicators and doses were prescribed to points.
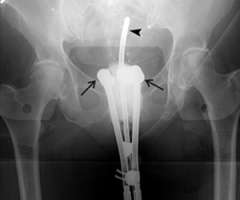
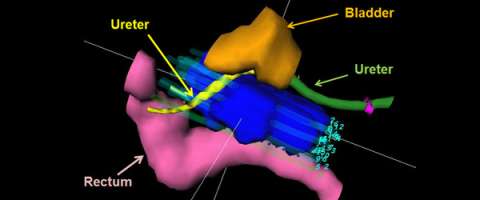
In the images below for example on the right is an anterior-posterior view of a vaginal applicator inserted in the vagina. You can see the cylinder pushing up against the top of the vaginal apex. You can't see the outline of the entire bladder and rectum but one way to approximate them is to place foley catheters in the bladder (yellow outline) and the rectum (brown outline) so they can be visualized.
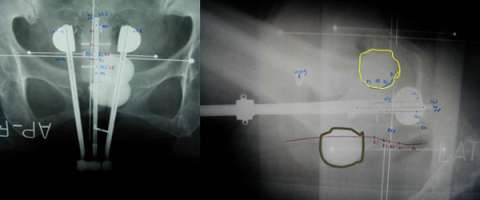
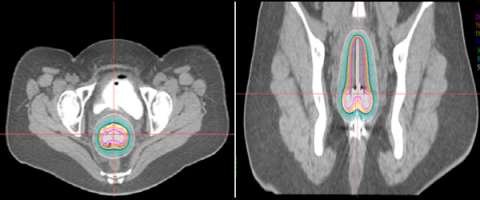
An alternative to this is to use CT compatible applicators and to use CT imaging. The advantage of this is that CT imaging allows you to see the anatomy in more detail than on X-rays. For example, a CT image with a vaginal applicator in place looks like the following:
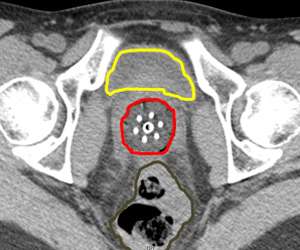
One can actually "see" the anatomy including the bladder (yellow) and rectum (brown). The cylinder is outlined in red. By being able to clearly see things we can more effectively optimize the radiation dose to the target and limit the dose to normal tissues.
Intracavitary
Intracavitary brachytherapy refers to when an applicator is placed directly into a "cavity". In gynecologic cancers this refers to placing an applicator in the vagina either up to the vaginal apex in cases where the cervix and uterus have been removed such as in endometrial cancer or through the cervical os and into the uterus for women being treated for cervical cancer.
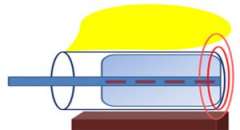
Below is a cartoon to help better illustrate an intracavitary applicator that might be used to treat the vaginal cuff. You can see the bladder in yellow, the vagina in the blue cylinder, the brachytherapy applicator in the light blue rectangle with the radiation dwell positions in red, and the rectum in brown. With a single channel applicator the radiation dose spreads out from the central part of the applicator essentially in circles. The most intense dose of radiation is nearest the radiation source and the radiation dose gets less intense the farther away from the source you go.
While most institutions only offer a single channel intracavitary applicator to treat the vaginal apex we offer multiple multichannel applicators as well as a single channel applicator. The multichannel applicators that we offer include:

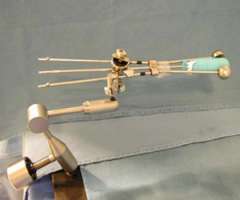

Our preference for using a multichannel applicator is based on research from our group published in 1999 that shows that a multichannel cylinder with 7 channels can reduce bladder and rectal doses by 15% or more than commonly used single channel applicators (Int. Journal Radiation Oncology, Biology, Physics, 1999). This work was based on 2-dimmensional planning and so we’ve redone this work with 3D-planning using a 13 channel applicator and once again show that multichannel applicators significantly reduce dose to the bladder and the rectum compared to single channel applicators. This data was presented at the World Congress of Brachytherapy in Barcelona in 2012 and was selected as one of the top 20 posters of the entire conference.
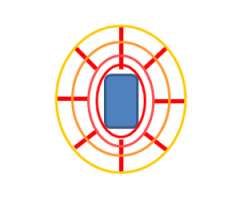
Below is a series of cartoons to help better illustrate the advantages of a multichannel applicator over a single channel one. The first image shows a radiation source (blue rectangle) and demonstrates that radiation is distributed equally in all directions from the radiation source. The most intense dose of radiation is nearest the radiation source (dark red circle) and the radiation dose gets less and less the farther away from the source you go.
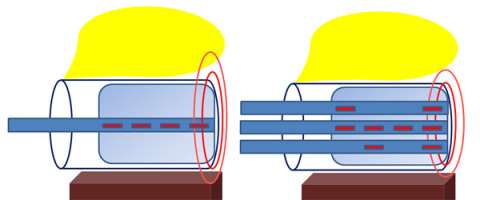
In the picture on the left you can see the bladder in yellow, the vagina in the blue cylinder, the brachytherapy applicator in the light blue rectangle with the radiation dwell positions in red, and the rectum in brown. With a single channel applicator the radiation dose spreads out from the central part of the applicator essentially in circles and because there is only one channel you can not alter the shape of this radiation distribution. So if there is an area of radiation dose that overlaps the bladder or rectum there is little that you can do about this.
In the picture on the left you can see an example of a 3 channel applicator. Based on computer programming we can figure out a patient specific pattern for the radiation dwell positions so we can keep more radiation dose off of the bladder and the rectum. We can accomplish this because having multiple channels gives us more dwell positions and therefore more flexibility in sculpting the radiation dose.
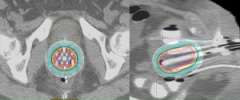
Below is an actual example of the CAPRI applicator (a 13 channel applicator) and the distribution of radiation dose that was delivered around the vaginal apex (right image axial and left image sagittal). Below to the right is a 3D depiction of the anatomy and distribution of the radiation dose (bladder in yellow, rectum in brown, applicator in purple, radiation dose in red):
Intracavitary applicators are also used to treat cervical cancer and the most commonly used applicator is a tandem and ovoid applicator.
Our approach at UCLA is to do 3D based instead of 2D based planning for these cases as well. Once again one can immediately appreciate how much additional detail in the anatomy and distribution of the radiation dose can be appreciated on the CT scan versus the X-ray.
Intractivity Treatment Process
Endometrial cancer
Intracavitary brachytherapy is commonly used in the post-operative treatment of endometrial cancer. The treatment is most commonly given either as 3 separate outpatient treatments usually 2 times per week or as 6 separate outpatient treatments given every other day. For the first treatment the patient lays down on her back on a CT scanner table. A vaginal cylinder is gently placed in the vagina by a physician and stabilized with a base plate. A CT scan is done of the pelvis to confirm that the cylinder is truly at the top of the vagina. Once this is confirmed then the end of the cylinder is connected via a transfer tube to the HDR afterloader. The HDR afterloader has a motorized cable with a small radiation source welded to the end of it. This radiation source is pushed to the top of the cylinder and delivers a radiation treatment over about 5-10 minutes. The source is then retracted back into the afterloader, the vaginal cylinder is removed and then the patient goes home. When the patient comes back for her subsequent treatments a repeat CT scan does not need to be performed and she will just proceed with having the applicator placed and then receiving the radiation treatment.
Cervical cancer
Brachytherapy treatment is a standard component of treatment for cervical cancer definitively treated with radiation. At our institution brachytherapy is most commonly given after completing external beam radiation therapy. It is most standardly given two times per week as an outpatient for a total of 5 treatments. Patients are given moderate sedation for the tandem and ovoid applicator placement. After the applicator is placed a CT scan or MRI is done to visualize the applicator relative to the patient's anatomy and residual tumor. Then a customized plan is generated. Once this is ready the tandem and ovoid applicator is connected via transfer tubes to the HDR afterloader. The HDR afterloader has a motorized cable with a small radiation source welded to the end of it. This radiation source is pushed to the top of the cylinder and delivers a radiation treatment over about 5-10 minutes. The source is then retracted back into the afterloader. The applicator is then removed and the patient goes home. The whole process typically takes 2-3 hours.
Interstitial
Interstitial refers to brachytherapy treatment where there isn't a cavity for a radiation applicator to fit into and so a series of small hollow little tubes are placed in and near the target tissue. An example of when this might be used is shown in the following illustrations. In the picture on the left there is an oval uterus narrowing down to the rectangular cervix which has a red cervical cancer and a larger rectangular vagina. Ideally after an initial course of external beam radiation therapy and concurrent chemotherapy this red cervical tumor will shrink and allow for a tandem and ovoid applicator (green figures) to fit into the vaginal fornices and cervix so the appropriate distribution of dose (orange) can be achieved (image on left).
Sometimes a lesion does not regress at the end of external beam radiation and may not allow the tandem and ovoid applicator to properly fit as in the picture on the right.
In other cases the tandem and ovoid applicator would fit but the residual lesion is larger than the standard distribution of dose with this applicator and so some of the tumor would be underdosed if this approach were used as is illustrated below on the left.

So in these cases an interstitial approach is best so that the residual disease can be appropriately encompassed as can be seen on the right.
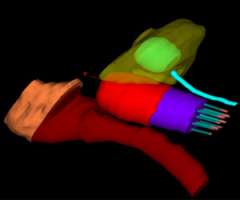
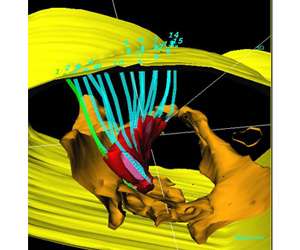
Below is a 3D rendering of the distribution of radiation dose and normal tissues from a gynecologic interstitial implant:
An axial CT slice showing the distribution of small hollow brachytherapy tubes and the distribution of radiation dose for a gynecologic interstitial brachytherapy implant.
Interstitial Treatment Process
These are cases that are performed in an operating room. The patient is given an epidural. Typically this will be combined with sedation for the case however some patients may undergo general anesthesia. In the operating room while you are asleep the small hollow tubes will be placed to cover the tumor. At the end of the case the hollow tubes will be stabilized through a plastic template that will be sutured to the skin. When you wake up you won’t be able to get up out of bed and walk around as the template would be displaced. So you need to remain in bed either on your back or on your side until all of the treatment is completed. After spending time in recovery you will be brought to the Radiation Oncology Department and a CT scan will be performed to verify the correct position of all of the tubes that were placed in the operating room. Then we will work on a customized plan. Once the plan is approved you will be treated up to two times per day until completing treatment. You will remain in the hospital the whole time that you are receiving this treatment.
Planning Session (simulation)
Vaginal cuff cylinder brachytherapy after surgery for endometrial or cervical cancer
About an hour prior to your simulation we will have you come to the clinic and the physician will place 3 small gold marker seeds near the vaginal apex. The purpose of doing this is so that the physician can easily identify the top of the vaginal apex on your CT scan. This is important because it helps us determine "how high" and "how low" to treat you.
**If you will be getting external beam prior to brachytherapy then you will already have marker seeds in place and you will not have to have this done again.
After the marker seeds are placed a foley catheter will be inserted into the bladder. This is done so that the bladder is drained for the simulation as well as so that we can place contrast into the bladder at the time of the simulation. This helps us better visualize it on the CT scan. Because a foley catheter is used we will write a prescription for an antibiotic (most commonly Cipro) to take once in the morning and once in the afternoon on the day of the simulation. In addition, it is best if you try to evacuate your bowels prior to coming to clinic.
After the marker seeds and foley catheter have been placed then you will be ready for the CT simulation.
Simulation for intracavitary cases
During this planning session you will be asked to lie on your back on a CT scanner. Your legs will be placed in stirrups and you will be positioned in the same way as you would be for a pelvic examination. The physician will then do a pelvic examination and evaluate which applicator will work best for your anatomy. The doctor will then gently insert the applicator into the vagina. After the applicator is properly inserted it will be stabilized. Your legs will then be brought down in the stirrups, contrast will be placed in the bladder via a foley catheter, and then a CT scan will be performed of the pelvis. The position of the applicator on the CT images will then be reviewed by your physician. If everything is positioned properly then the applicator will be removed, your legs will be brought down from the stirrups, and the simulation will be completed. If the applicator is not in the ideal location then it will be adjusted and another CT scan of the pelvis will be performed to verify its position. This entire simulation process takes about 1 hour. No intravenous contrast is used during this simulation.
Simulation for interstitial cases
This planning session is similar to the intracavitary one except for the fact that the small hollow tubes that are used for interstitial cases are placed in the operating room and then you are brought down to the Radiation Oncology Department for your CT simulation as opposed to having the applicator placed in the CT simulation room as is done with intracavitary cases.
Once you are brought to the CT simulation room you will be transferred from a gurney to the CT table. You will be lying down flat on your back for the simulation. Contrast will be placed in the bladder and the rectum. A CT scan will then performed of the pelvis. The images will be reviewed by your physician and he/she will decide whether any adjustments to the hollow tubes are needed. If adjustments are necessary then they will be made and another CT scan will be performed to assess the final location of the hollow tubes. This process takes about 1 hour. No intravenous contrast is used during this simulation. After the simulation you will be taken off of the CT table and a therapist will do some measurements of each of the hollow tubes that have been placed. These measurements are used to help us with treatment planning.
What happens after the simulation is done?
Intracavitary cases
Treatment can start the following day after the simulation. Treatment is typically given either two times per week (with at least 48 hours in between treatments) or every other day. Usually 3-6 treatments are given depending on the clinical scenario.
Interstitial cases
Treatment will start either later the same day as the simulation or the following morning. Treatments are standardly given two times per day (once in the morning and once in the afternoon).
Is there anything that I need to do prior to each treatment?
Intracavitary
You will be asked to empty your bladder prior to the insertion of the applicator and to the best of your ability try to stay regular with your bowel movements so that you don't get constipated or have too much gas.
Interstitial
You will be an in-patient for this treatment so there is nothing extra that you need to do prior to your treatments.
What happens when I come in for the actual treatment?
When you come in for your simulation before you leave you will be given a schedule with your treatment start date and time. For brachytherapy we start treatment at 8 AM and complete treatment at around 6 PM.
Intracavitary
You will be set-up in the same position as at the time of simulation. For the actual treatment you will not have marker seeds placed again and you will not have a foley catheter in the bladder. The applicator will be placed gently and secured into place. A CT scan will be performed to verify the position of the applicator. If minor adjustments need to be made they will and a CT will be done again. Once the applicator is in the proper position you will be transferred while in stirrups and with the applicator in place onto a gurney. You will then be transported next door to the brachytherapy suite and the applicator will be connected to the HDR remote afterloader. The doors will be closed (there is a camera and microphone in the room so you can communicate with us) and then treatment will begin. During the treatment you will hear the motor from the afterloader pushing and pulling the radiation source in and out of each of the channels of the applicator. You won’t feel or see the radiation being delivered. The treatment will take about 5-10 minutes. Once the treatment is done you will be disconnected from the afterloader and the applicator will be removed. This whole process takes about 1-1.5 hours.
Interstitial
The position of the hollow catheters will be confirmed on the CT scanner or with fluoroscopy prior to your treatment being delivered to ensure that nothing has moved out of place. If it has then minor adjustments will be made. Once this has been done you will be transported to the brachytherapy suite and the implant will be connected to the HDR remote afterloader. The doors will be closed (there is a camera and microphone in the room so you can communicate with us) and the treatment will then begin. During the treatment you will hear the motor from the afterloader pushing and pulling the radiation source in and out of each of the channels of the applicator. You won’t feel or see the radiation being delivered. The treatment will take about 10-15 minutes. Once the treatment is done you will be disconnected from the afterloader.
Information for patients preparing for gynecologic brachytherapy
- Preparing for your Outpatient Brachytherapy Procedure at the Ambulatory Surgery Center Peter Morton Medical Building (200 UCLA Medical Plaza) in Suite 660
- Preparing for Surgery at Ronald Reagan UCLA Medical Center
- Gynecologic (GYN) Brachytherapy information brochures:
UCLA publications on gynecologic brachytherapy
Moving from standardized to personalized boxes and pears in radiation planning for cervical cancer.
Mesko S, Kamrava M.
Curr Opin Obstet Gynecol. 2016
Early clinical outcomes of ultrasound-guided CT-planned high-dose-rate interstitial brachytherapy for primary locally advanced cervical cancer. Mesko S, Swamy U, Park SJ, Borja L, Wang J, Demanes DJ, Kamrava M.
Brachytherapy. 2015
The ideal adjuvant treatment in node positive vulvar cancer is (fill in your best guess here).
Kamrava M.
Gynecol Oncol. 2015
Ureteral stent insertion for gynecologic interstitial high-dose-rate brachytherapy.
Demanes DJ, Banerjee R, Cahan BL, Lee SP, Park SJ, Fallon JM, Reyes P, Van TQ, Steinberg ML, Kamrava M.
Brachytherapy. 2015
Potential role of ultrasound imaging in interstitial image based cervical cancer brachytherapy.
Kamrava M.
J Contemp Brachytherapy. 2014
Brachytherapy in the treatment of cervical cancer: a review.
Banerjee R, Kamrava M.
Int J Womens Health. 2014
Dosimetric comparison of 3-dimensional planning techniques using an intravaginal multichannel balloon applicator for high-dose-rate gynecologic brachytherapy.
Park SJ, Chung M, Demanes DJ, Banerjee R, Steinberg M, Kamrava M.
Int J Radiat Oncol Biol Phys. 2013
Electronic brachytherapy for postsurgical adjuvant vaginal cuff irradiation therapy in endometrial and cervical cancer: a retrospective study.
Kamrava M, Chung MP, Demarco J, Kayode O, Park SJ, Borja L, Chow L, Lee SP, Steinberg ML, Demanes DJ.
Brachytherapy. 2013
American Brachytherapy Society consensus guidelines for interstitial brachytherapy for vaginal cancer.
Beriwal S, Demanes DJ, Erickson B, Jones E, De Los Santos JF, Cormack RA, Yashar C, Rownd JJ, Viswanathan AN; American Brachytherapy Society.
Brachytherapy. 2012
American Brachytherapy Society consensus guidelines for adjuvant vaginal cuff brachytherapy after hysterectomy.
Small W Jr, Beriwal S, Demanes DJ, Dusenbery KE, Eifel P, Erickson B, Jones E, Rownd JJ, De Los Santos JF, Viswanathan AN, Gaffney D; American Brachytherapy Society.
Brachytherapy. 2012
American Brachytherapy Society consensus guidelines for locally advanced carcinoma of the cervix. Part II: high-dose-rate brachytherapy.
Viswanathan AN, Beriwal S, De Los Santos JF, Demanes DJ, Gaffney D, Hansen J, Jones E, Kirisits C, Thomadsen B, Erickson B; American Brachytherapy Society.
Brachytherapy. 2012
High dose rate transperineal interstitial brachytherapy for cervical cancer: high pelvic control and low complication rates.
Demanes DJ, Rodriguez RR, Bendre DD, Ewing TL.
Int J Radiat Oncol Biol Phys. 1999
The use and advantages of a multichannel vaginal cylinder in high-dose-rate brachytherapy.
Demanes DJ, Rege S, Rodriquez RR, Schutz KL, Altieri GA, Wong T.
Int J Radiat Oncol Biol Phys. 1999
Options for Women with Recurrent Malignancies
Patients with gynecological malignancies who have received prior radiation or have recurrent malignancies present significant challenges to providers. Options for treatment have traditionally been limited to surgery; however, this can carry significant morbidity.
Here at UCLA we have specialized treatments that can help in difficult situations like this. The modality that is selected depends on multiple factors including the location of the recurrence, the size of the recurrence, the patient’s previous treatments, and the patients overall health.
Our team has extensive experience in something that few other departments can do which is interstitial brachytherapy. This is a highly skilled technique were a series of hollow tubes are inserted through the skin to encompass the area of recurrence. These hollow tubes serve as a matrix that allows a small source of radiation on the end of a cable to deliver radiation in a very precise way directly to the target. This type of treatment is called High Dose Rate brachytherapy. It is a highly effective and useful treatment in many different settings.
Not only can HDR brachytherapy be done by our team but it can also sometimes be used in collaboration with the Gynecologic Oncology team. There are situations were not all of the tumor can be taken out and a focused amount of radiation to this residual area is necessary. At the time of the operation we can place these hollow tubes so that we can post-operatively deliver focused doses of radiation.
Another treatment option that is available is stereotactic body radiation therapy (SBRT). This is a technically advanced treatment that allows us to safely and confidently deliver focused doses of radiation to a target while dramatically reducing additional radiation dose to surrounding tissues. Here at UCLA we have been using SBRT to treat multiple body sites including lung, liver, and pancreas. We have also now started to use this is selected cases for patients with gynecological cancers.


Gynecologic Oncology

Medical Oncology

John A. Glaspy, MD
