Chimeric Antigen Receptor (CAR) T-cell Therapy
CAR T-cell therapy, a form of immunotherapy, targets and destroys blood cancer cells.
Immunotherapy helps patients fight different forms of cancer by enabling the immune system to both recognize and attack cancer.
UCLA Health physicians are at the forefront of immunotherapy for cancer. As leaders in CAR T-cell therapy, we pioneered these groundbreaking treatments and continue to make them more effective.
What is CAR T-cell Therapy?
CAR T-cell therapy activates a person’s immune system to fight off cancer. This groundbreaking treatment is a type of immunotherapy called effector cell therapy or engineered cell therapy. Immunotherapy is still relatively new, but it shows promise for adults and children for whom other treatments have stopped working.
T-cells are the central components of the immune system. In CAR T-cell therapy, T-cells are collected from the patient’s blood. Then, in a laboratory, specialists modify the T-cells so they can recognize and attack certain blood cancers.
The modified cells are then returned to the patient’s body. If the treatment is successful, the newly modified T-cells will help a person’s immune system kill off cancer cells, sending the disease into remission.
How Does CAR T-cell Therapy Work?
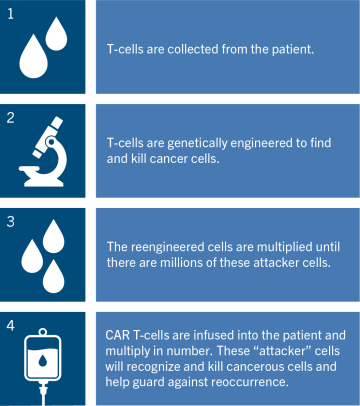
T-cells (a type of white blood cell) are the central components of the immune system. In CAR T-cell therapy, T-cells are collected from the patient’s blood. Then, in a laboratory, specialists modify the T-cells so they can recognize and attack certain blood cancers.
The modified cells are then returned to the patient’s body. If the treatment is successful, the newly modified T-cells will help a person’s immune system kill off cancer cells, sending the disease into remission after only one treatment.
What Can You Expect Before, During & After CAR T-cell Therapy?
As you begin CAR T-cell therapy, remember that you’re in good hands. UCLA Health specialists have delivered more than 200 infusions of FDA-approved CAR T-cell therapy since 2018.
Before CAR T-cell therapy:
- Activate your myUCLAhealth (MyChart) account
- Download the MyChart app to your smartphone
- Complete tasks assigned to you in myUCLAhealth (MyChart), including daily symptom tracker tasks
- Read education materials
- Meet with a social worker to discuss a plan for your care while receiving CAR T-cell therapy, including local lodging and transport needs
- Complete an Advanced Care Planning appointment with a nurse practitioner (adults only)
- Attend all appointments and screening tests to prepare for CAR T-cell therapy
- Identify at least one caregiver
- Review and sign consent forms with your health care provider
- Identify alternative transportation for at least eight weeks after your CAR T-cell therapy; you cannot drive or operate heavy machinery for at least eight weeks
Your health care provider will collect the T-cells from your body using a process called apheresis. During apheresis, a machine separates your blood into different parts, with the goal of only removing the T-cells. The remaining blood is returned to your body. Your T-cells may be collected from your arm veins or from a catheter, which is placed before the collection starts.
The T-cells are sent to a laboratory where they are modified to attack cancer cells. The new T-cells are kept in the laboratory to grow and multiply. Once enough have been made, they are frozen until they are ready to be sent to UCLA Health.
During CAR T-cell therapy:
- Lymphodepletion: You will undergo chemotherapy treatment to prepare your body for the infusion of the T-cells. This treatment usually takes place in our outpatient clinic. You will have a catheter (also known as a central line) placed before lymphodepletion, which will allow your health care team easy access to give you medications and fluids. The catheter will be kept in place for at least 28 days or longer if needed.
- Hospital/clinic admission: Once the chemotherapy is complete, you will be admitted to the clinic or hospital. Your care team will determine the location. Your team will review the details for your CAR T-cell infusion. You will be monitored closely by your team for at least seven to 14 days. While in the hospital, you will be monitored closely. You may be connected to continuous cardiac and/or pulse oximeter monitoring.
- CAR T-cell infusion: Day 0 is the day of the infusion of the CAR T-cells and is used as a starting point for counting how far out you are from receiving them. For example, the day after the infusion would be Day 1, the next Day 2, and so on.
After CAR T-cell therapy:
After infusion, the T-cells will continue to multiply to target and attack your cancer cells. Your safety is our top priority. Once you have received your infusion, your care team will monitor you closely for possible side effects and provide treatment if needed.
Patient Education Resources
The CAR T-cell Therapy Guide provides information on CAR T-cell therapy for patients, caregivers and families, including a patient checklist, list of caregiver expectations, and information on potential side effects of CAR T-cell therapy and details on the recovery process. The guide is presented as both a digital flip book and downloadable PDF, and is available in English and Spanish.
CAR T-cell Therapy Guide
CAR T-cell Therapy Side Effects
Cytokine Release Syndrome: In some patients, substances called cytokines are released by the body in response to an overactive immune system. Patients may experience a high fever or chills, headaches, nausea, vomiting, diarrhea, dizziness, low blood pressure, difficulty breathing or confusion.
Neurologic: In rare cases after CAR T-cell infusion, the brain and neurologic system are temporarily altered. Patients may experience confusion, difficulty talking or memory loss, headaches, seizures, tremors or loss of consciousness.
Note: Most CAR T-cell therapy side effects are temporary and reversible.
Who is a Candidate for CAR T-cell Therapy?
The FDA currently has approved six CAR T-cell therapies to treat blood cancers. It is offered to select patients who have:
| Cancer Type | Patient Criteria | Treatment Type* |
|---|---|---|
|
Diffuse large B-cell lymphoma (DLBCL) |
Adult patients who have relapsed or not responded to previous treatments. |
Breyanzi (lisocabtagene maraleucel) Kymriah (tisagenlecleucel) Yescarta (axicabtagene ciloleucel) |
|
Acute lymphoblastic leukemia (ALL) |
Children and young adults up to age 25 who have relapsed or not responded to previous treatments. |
Kymriah (tisagenlecleucel) |
|
Acute lymphoblastic leukemia (ALL) |
Adult patients who have relapsed or not responded to previous treatments. |
Tecartus (brexucabtagene autoleucel) |
|
Mantle cell lymphoma (MCL) |
Adult patients who have relapsed or not responded to previous treatments. |
Tecartus (brexucabtagene autoleucel) |
|
Follicular lymphoma (FL) |
Adult patients who have relapsed or not responded to previous treatments. |
Yescarta (axicabtagene ciloleudel) |
|
Multiple myeloma (MM) |
Adult patients who have relapsed or not responded to previous treatments. |
Abecma (idecabtagene vicleucel) Carvykti (ciltacabtagene autoleucel) |
*Your doctor will help you determine the best CAR T-cell therapy for you.
Why Choose UCLA Health for CAR T-cell Therapy?
Immunotherapy helps patients fight different forms of cancer by enabling the immune system to both recognize and attack cancer. One form of immunotherapy, chimeric antigen receptor (CAR) T-cell therapy, specifically targets blood cancers so they can be eliminated.
The physicians and researchers at UCLA Health are at the forefront of immunotherapy innovation. Highlights of our program include:
- Groundbreaking treatments: In 2018, UCLA Health became one of the first health systems in the nation to offer commercial CAR T-cell therapy. In 2020, UCLA Health was one of the first to offer Food & Drug Administration (FDA)-approved CAR T-cell therapy to treat relapsed or refractory mantle cell lymphoma. We previously offered this therapy to participants in clinical trials.
- Experience and expertise: Our outstanding team includes experts in hematology, oncology, and blood and bone marrow transplantation. Using a coordinated approach, we work closely with experienced researchers to deliver groundbreaking cancer treatments through immunotherapy clinical trials and treatments.
- Clinical trials: The physicians and researchers at the UCLA Health Jonsson Comprehensive Cancer Center (JCCC) lead the way in cancer research. If you're eligible, you'll have access to a variety of immunotherapy clinical trials. Your doctor will discuss open CAR T-cell therapy trials currently available and help you assess the risks and benefits of treatment. Find a clinical trial.
- Pioneering the latest therapies: UCLA Health specialists are consistently prepared to deliver new treatments as soon as they're approved by the FDA. We stay on top of the latest developments so we can offer effective treatments the moment they're available.
Locations
Infusion therapy: Offered at both Ronald Reagan UCLA Medical Center and the UCLA Santa Monica Medical Center.
Consultation & Clinic: Appointments are scheduled in Westwood at Bowyer Oncology Center and Westwood Pediatrics, and in Santa Monica at Santa Monica Cancer Care (2020).
Adult Program
Pediatric Program
Contact Us
For more information about CAR T-cell therapy at UCLA Health, please call 310-206-6909.
Find your care
UCLA Health immunotherapy specialists develop a personalized plan to treat blood cancer. For more information, call our cancer services team at 310-206-6909.
Helpful Links
Updates in CAR T-Cell Therapies for Blood Cancer Treatment
Sarah Larson, MD | Closer to a Cure Cancer Series