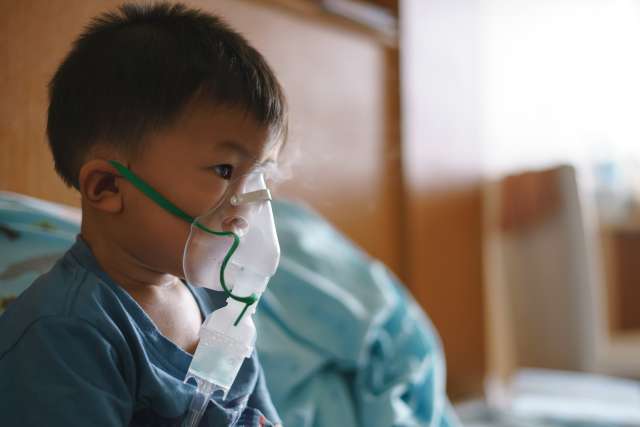
Andreas Schwingshackl, MD, PhD, Associate Professor of Pediatrics and Chief of the Pediatric Critical Care division at Mattel Children’s Hospital, is bullish on research. Having trained in research in Austria and Canada prior to commencing his clinical career, he continues to flex his research capability at UCLA.
“We are working on acute lung injury and acute respiratory distress syndrome (ARDS), which has been around for a very long time,” says Dr. Schwingshackl.
Before COVID-19 appeared, most people who died from ARDS actually died from influenza-induced ARDS. That changed during the pandemic, to deaths from COVID-19-induced ARDS, but influenza cases may be on the rise again, notes Dr. Schwingshackl.
“We will have to see how this winter goes.”
The UCLA team is trying to develop new anti-inflammatory therapies against ARDS by targeting electrophysiological properties of cells in the lung.
“We are looking at electrolyte fluxes – like sodium, potassium and calcium fluxes – that go across the cell membrane of lung cells,” explains the clinician researcher.
In addition to mimicking ARDS in mice models, researchers also work with human lungs from donors who died from lung injury.

“We examine and isolate cells from the human donor lungs,” says Dr. Schwingshackl. He and his colleagues work with biomedical engineering departments around the country to bioengineer lung models with clinically relevant features of human lungs.
“Models, using donor lung cells, allow us to study processes in inflamed lungs,” says Dr. Schwingshackl.
“For example, the most common therapy for any lung disease is oxygen. So, we need to be able to expose our experimental models to high oxygen concentration, just as would happen in an emergency room. Additionally, because lungs move constantly, inhaling and exhaling, we must be able to stretch the model cells and tissue constantly. The models also need to allow us to give medications and other drugs to the cells and tissue all at the same time.”
The resulting models are three-dimensional stretchable structures coated with human cells.
“Some surfaces are exposed to air like the inside of the lung. And then on the opposite side we have liquid, and cells that come from a blood vessel, just like in the bloodstream,” says Dr. Schwingshackl.
The work, ongoing for 15 years, has helped to identify some compounds that affect potassium currents across the cell membrane in a very particular and specific way. This work has been ongoing in a collaboration with Dr. Minor, a biochemist at the University of California San Francisco.
“We have been injecting these compounds into mice infected with influenza virus or bacteria for several years. We put them under high oxygen concentrations and then, as crazy as it sounds, we put the mice on breathing machines,” says Dr. Schwingshackl.
“We administer the new compounds through their breeding tubes. We have been very successful in improving pneumonia and inflammation in the mice’s lungs. The next step would be to go into larger animals.
“We are hopeful that in the next five-to-ten years, we can start talking about Phase One trials in humans. That is an exciting thought.”
Related:
Ensuring community access to the highest level of pediatric critical care