A portable, totally man-made artificial kidney that is small enough to fit inside a backpack is potentially a game-changer for patients with renal failure.
The device is being developed by Dr. Ira Kurtz, MD, FRCP, FASN, chief of the UCLA Health Division of Nephrology, in partnership with the U.S. Kidney Research Corp. The team includes Roland Ludlow, CEO of U.S. Kidney Research Corp. and Jamie Hestekin, PhD, a researcher from the University of Arkansas.
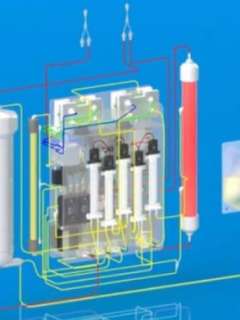
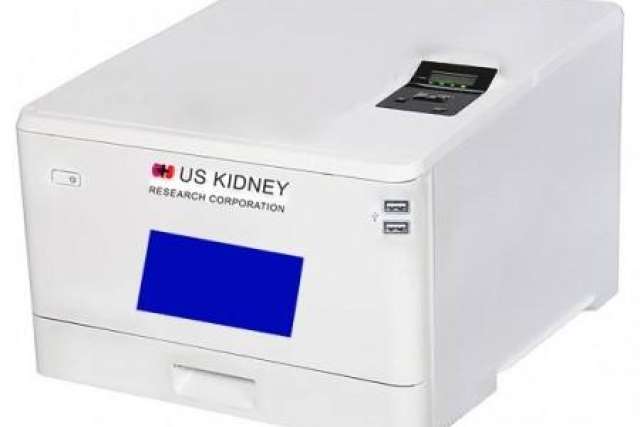
Their hope is that the waterless, dialysate-free technology developed for this new device will lead to the creation of the first implantable artificial kidney within six years.
“Ours is the only project that simulates the essential functions of the kidney in one device,” Dr. Kurtz says.
For their efforts, Dr. Kurtz and his team have been awarded the 2021 Artificial Kidney Prize by The Kidney Innovation Accelerator (KidneyX), a public-private partnership between the U.S. Department of Health and Human Services (HHS) and the American Society of Nephrology (ASN) to accelerate innovation in the prevention, diagnosis and treatment of kidney diseases.
The accolades don’t stop there. Last year, General Electric Co. named the team’s artificial kidney device among “The Five Coolest Things on Earth.”
“It’s a fantastic honor,” Dr. Kurtz says about the KidneyX award. “It’s something that puts a cap on the work we’ve been doing for the past six years, and it gives national and international recognition we wouldn’t have otherwise received.”
Aside from personal fulfillment, Dr. Kurtz says, the KidneyX award creates greater awareness of the project and will enable his team to obtain necessary funding — about $9 million more over the $3.5 million that already has been spent — to produce the final prototype so pig trials can begin in 20 months and clinical trials in three years.
100% man-made
Unlike competing artificial kidney projects, Dr. Kurtz’s prototype uses completely man-made rather than living components to replicate the key functions of the kidney. He believes this is a more realistic approach to achieving their final goals.
He notes that the kidney is composed of approximately 45 different cell types that have unique functional properties.
“In other programs that are attempting to use living cells, no one has ever taken the required cells out of the kidney and shown that their electrolyte transport functions are completely normal outside the kidney for any length of time. The cells recognize they are not in their native environment and rather quickly alter their properties. That’s a major impediment,” Dr. Kurtz says.
He likens his team’s approach to that of the Wright brothers: While others were trying to build a flying machine that attempted to simulate the beating of a bird’s wings, the brothers worked out the key engineering principles of what is needed to fly.
Dr. Kurtz envisions the development in three phases: Phase 1 is the portable device that will be placed on a desk or night table; Phase 2 is a smaller artificial kidney device that can be carried on a person attached to their belt; and Phase 3 is to reduce the size of the device even further to make it fully implantable.
Benefits of the device will include:
- Increased mobility and ability to travel and work longer hours
- Diet and fluid intake potentially liberalized
- Instant treatment data for patients and caregivers
- No need for forearm access or anti-rejection meds with the implantable device
- The opportunity to get off dialysis
For those patients remaining on dialysis, the technology can also can be used in conjunction with peritoneal dialysis (a type of self-dialysis that is done daily at home) or hemodialysis to decrease the amount of dialysis solutions needed, Dr. Kurtz says.
An important feature of the artificial kidney device is that it does not use water, dialysate solutions or dialyzers, eliminating the need for large reverse osmosis water tanks and large storage space for home modalities. This potentially will save billions of gallons of water each year, he says.
The device also will reduce the carbon footprint associated with generating purified water, manufacturing dialysate solutions and manufacturing dialyzers, he adds.
How it works
At the outset, Dr. Kurtz and his colleagues set out to design an artificial kidney that provides key complex functions that simulate the kidney’s filtration and transport properties — not a simple task, he says.
The kidney maintains blood chemistry, which is constantly changing based on food and water intake, he explains. The kidney recognizes that and continually makes adjustments to keep one’s blood chemistry constant.
“It’s really a number of separate functionalities put into one organ. In this regard, creating an artificial kidney is much more complicated than designing an artificial heart or a pancreas, where one is essentially replicating a single organ function,” Dr. Kurtz says.
The artificial kidney device consists of four separate types of components:
- Ultrafiltration module, which is a sophisticated filter that prevents the filtration of blood cells and proteins while sufficiently filtering water, urea and electrolytes
- Nanofiltration module, which is also a filter but has the properties of preventing glucose excretion and allowing the permeation of urea, electrolytes and water
- Electrodeionization modules, which use an electric current to drive the transport of specific electrolytes from one solution to another
- Reverse osmosis module, which transfers water from the synthetic urine stream to the blood so that the synthetic urine volume approximates the patient’s water intake
Built into the device is dynamic feedback control capability — software that ensures various functions are not fixed but can change to compensate for alterations in dietary food and water intake.
Dr. Kurtz says the team has made significant advancements in the ultrafiltration module’s properties since the onset of development.
“A major problem with having a filter is that although it can function properly initially, over time the capacity of the filter decreases. Filters tend to get fouled by proteins in the blood or there is clotting, both of which decrease the capacity of the ultrafiltration process over time. To prevent these phenomena from occurring is hard to accomplish,” he says.
The current prototype uses new ultrafiltration membrane chemistry that has significantly less fouling, less clotting, and that also has filtration properties that exceed anything currently being manufactured, Dr. Kurtz says.
Further, the electrodeionization modules have been modified so they can transport specific electrolytes individually rather than coupled together, as the commercial ones do now, he says.
Solves a growing problem
Some 660,000 Americans currently are living with renal failure, and 100,000 are waiting for a kidney transplant, according to statistics from the National Kidney Foundation.
In addition, approximately 100,000 Americans begin dialysis each year, and one-in-five of them will die within a year, Dr. Kurtz says.
The artificial kidney device will fill a growing need for a replacement for dialysis, he says. “This device will allow patients to become much freer and more able to take more charge of their life and not focus as much psychologically on their treatment that currently takes over their lives.”
Current clinical approaches to kidney failure include hemodialysis, peritoneal dialysis and kidney transplantation. Dialysis is not an optimal therapy because patients suffer emotionally and medically, Dr. Kurtz says.
He notes that hemodialysis requires 3½-hour treatments three times a week, which does not come close to replicating the 24/7 function of a normal kidney. Nor does peritoneal dialysis, he adds.
Transplantation is not an option for many patients due to a donor/organ shortage, he says.
As the population ages, the problems will worsen, Dr. Kurtz says. “We keep getting better at delaying the onset or slowing down the progression of kidney disease, so our patients tend to get older.”
With aging comes problems of depression and lack of mobility — about 15% to 20% of patients have to receive transportation to a dialysis unit, he says.
Dr. Kurtz notes that kidney disease care cost the U.S. government $114 billion last year; about 15% of the $776 billion Medicare budget in 2020. The $30,000 to $50,000 cost for the artificial kidney device will represent a substantial saving to Medicare, he says.
“Congress is trying to cut the expense in this area, and one push is to fund innovation to develop an alternative to kidney dialysis,” he says.
Dr. Kurtz believes his artificial kidney will be the answer.
For more information on the Division of Nephrology at UCLA Health, visit uclahealth.org/nephrology
Jennifer Karmarkar is the author of this article.




