Physicians Update
Winter 2022
Included in this issue:
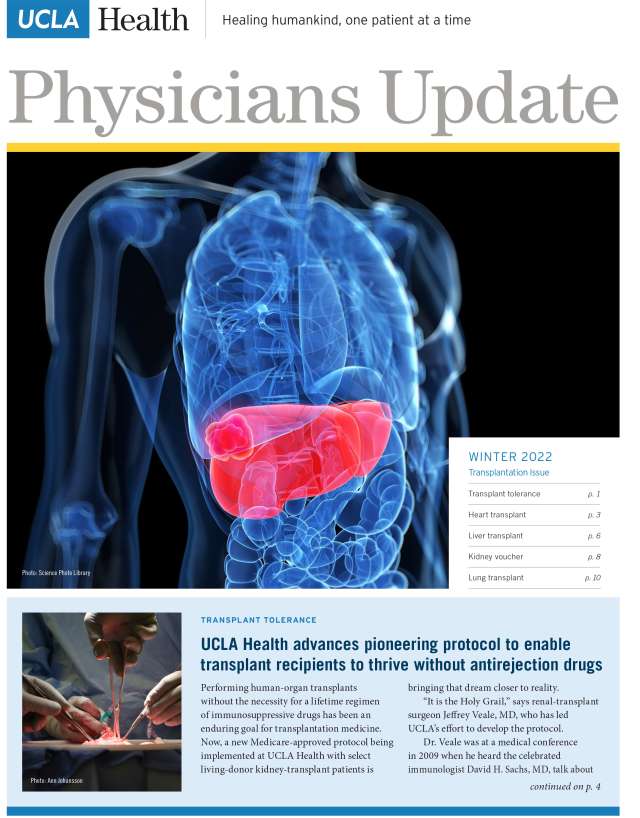
- UCLA Health advances pioneering protocol to enable transplant recipients to thrive without antirejection drugs
- Multidisciplinary care at the heart of UCLA’s adult heart-transplant program
- UCLA program improves outcomes for patients with rare liver cancer
- Vouchers expand reach of living-kidney donations
- UCLA’s lung-transplant program demonstrates success with highest-risk patients
- UCLA Advanced Lung Disease and Lung Transplantation Symposium
Spring 2021
Included in this issue:
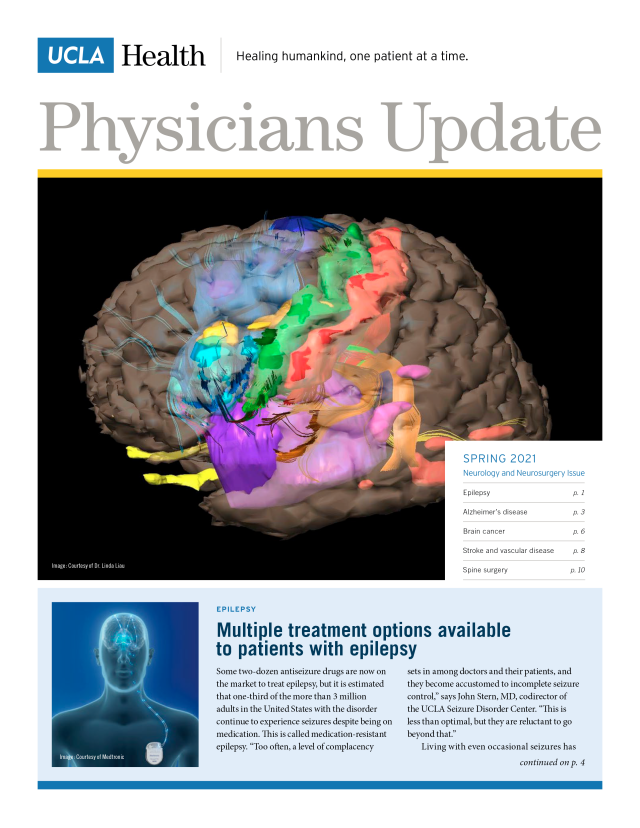
- Multiple treatment options available to patients with epilepsy
- Patients with dementia benefit from treatment in specialized setting
- UCLA Brain Tumor Center takes multidisciplinary approach to attacking glioblastoma
- Comprehensive care following stroke is vital to fullest possible recovery
- Advances in spine surgery open door to relief for a broader spectrum of patients

Winter 2020
Included in this issue:
- Less-invasive approaches to repairing and replacing heart valves expands scope of therapies for more patients
- Sports cardiology program tailors care to competitive athletes
- New clinic provides personalized care for patients with inherited or genetic cardiovascular disease
- Mechanical circulatory support has extended life-sustaining options for advanced heart failure patients
- UCLA Cardiac Arrhythmia Center on the forefront of care for patients with most challenging conditions
- UCLA Heart Failure Symposium 2020

Fall 2020
Included in this issue:
- Anesthesiologists have significant role to play in addressing opioid crisis
- Clinical Updates
- Regional anesthesia a boon to patients having joint replacement surgery
- The role of anesthesiologists changes with the times
- New technologies advance pain care for patients with cancer
- Standardized protocols improve outcomes for patients across a broad spectrum of surgical procedures

Fall 2019
Included in this issue:
- Herceptin has saved countless women’s lives and earned UCLA’s Dr. Dennis Slamon the Lasker Award
- Three-drug combination helps curb the growth of a deadly type of skin cancer
- CAR T clinical trial aims at extending the lives of people with most common types of lymphoma and leukemia
- Promise of CAR T-cell therapy may extend beyond current uses
- 3D modeling to prepare for cancer surgeries should become new standard
- Shorter course of radiation therapy effective in treating men with prostate cancer
- Center works to identify children with genetic predisposition to cancer
- Adding ribociclib to hormone therapy extends lives of women with most common breast cancer
- BRCA initiative aims to increase access to testing

Spring 2019
Included in this issue:
- Center works to identify children with genetic predisposition to cancer
- Specialized center offers evaluation and treatment for perplexing bone disorders
- Drug study aims to reduce risk of preterm labor in women with infection in the womb
- New therapies lead to major advances in treatment of cystic fibrosis
- Children with short bowel syndrome benefit from recent treatment advances
- 4th Annual Pediatric Board Review Course/Board Prep Course

Fall 2018
Included in this issue:
- Advances in facial reanimation are restoring smiles
- Center is a national resource for treating the full range of voice problems, from common to complex
- Researchers explore pharmacological approaches to treat NF2 tumors
- New chair of head and neck surgery looks to the future
- Taking steps toward personalized treatment for patients with head and neck cancers
- Program established to address needs of head and neck cancer survivors
- UCLA center offers management of complex nasal and sinus problems
- Multidisciplinary expertise is best approach to treating balance disorders

Spring 2018
Included in this issue:
- Campaign aims to boost rates of colon cancer screening
- MRI, endoscopy being used to screen patients at elevated risk for pancreatic cancer
- Hands-on, multidisciplinary approach benefits IBD patients
- Addressing diet and nutrition is essential when treating GI disorders
- POEM procedure offers relief for patients with achalasia
- Vatche and Tamar Manoukian Division of Digestive Diseases
- UCLA Clinical Updates Spring 2018

Winter 2018
Included in this issue:
- Specialized epilepsy center offers potential remedies to patients with uncontrolled seizures
- New center offers comprehensive care for teens with epilepsy
- Hospital on wheels brings immediate care to stroke patients
- Addressing sleep issues can aid diagnosis of health concerns
- Advances in neurogenetics opens window to rare neurological conditions
- 6th Annual UCLA Review of Clinical Neurology

Winter 2018
Included in this issue:
- Specialized epilepsy center offers potential remedies to patients with uncontrolled seizures
- New center offers comprehensive care for teens with epilepsy
- Hospital on wheels brings immediate care to stroke patients
- Addressing sleep issues can aid diagnosis of health concerns
- Advances in neurogenetics opens window to rare neurological conditions
- 6th Annual UCLA Review of Clinical Neurology

Fall 2017
Included in this issue:
- UCLA Registry Helps Researchers and Physicians to Better Understand Dwarfism
- Synthetic-Cartilage Implant Offers Relief from Great Toe Arthritis
- Tailoring Sarcoma Care to Meet the Needs of the Patient
- New Orthopaedic Surgery Chair Addresses the Challenges Ahead
- Partnership with Lakers Yields Benefits for Everyday Athletes
- 8th Annual UCLA Musculoskeletal Ultrasound Course and Hands-on Workshop

Spring 2017
Included in this issue:
- New Chemo Vector Offers Option for Patients with Difficult to Treat Urologic Cancer
- Immunotherapy Treatment Can Increase Survival for Some Patients with Prostate Cancer
- UCLA Clinic Takes Holistic Approach to Men’s Health
- Comprehensive Approach Needed to Address Recurrent UTIs
- UCLA Radiation Oncology Workshop: Integration of State-of-the-Art Innovations into Clinical Radiation Oncology Practice