Drs. Lueckerath/Mona Lab
Nuclear Medicine Pre-Clinical Team
Our group focuses on theranostic approaches with the goal to deliver new insights into tumor biology, heterogeneity and metabolism and their relationship and relevance to functional imaging and biomarker-driven treatments in nuclear medicine.
Theranostics
Theranostics use radioligands that bind to cell surface receptors on target cells. Radioligands labeled with isotopes such as Gallium-68 enable imaging of target expression by positron emission tomography (PET). The same or a similar ligand labeled with alpha or beta radiation emitting isotopes (e.g., Actinium-225, Lead-212; Lutetium-177, Yttrium-90,) delivers therapeutic radiation specifically to target expressing tissues (radionuclide therapy; RNT). In contrast to conventional radiotherapy, RNTs are systemically administered; as a consequence, metastases throughout the body can be targeted, and tumors are irradiated for hours to days.
Research Focus
Radionuclide therapy is a promising treatment option for various cancers. However, outcomes are rarely curative. We address this clinical need by investigating the mechanisms underlying the limited effectiveness of RNT. These mechanisms include, for example, tumor cell intrinsic resistance mechanisms, tumor protection by microenvironmental factors, and a suboptimal radiotherapeutic strategy. Poor understanding of these mechanisms represents a key barrier to the development of more effective RNT approaches. To improve radionuclide therapy outcomes and to establish rationally chosen, translatable RNT-based combination therapies, we characterize cancer mouse models and patient samples, and use diverse in vitro, ex vivo, and in vivo analyses to identify targetable RNT-induced alterations in tumor, stroma and immune cells that mitigate the effects of RNT.
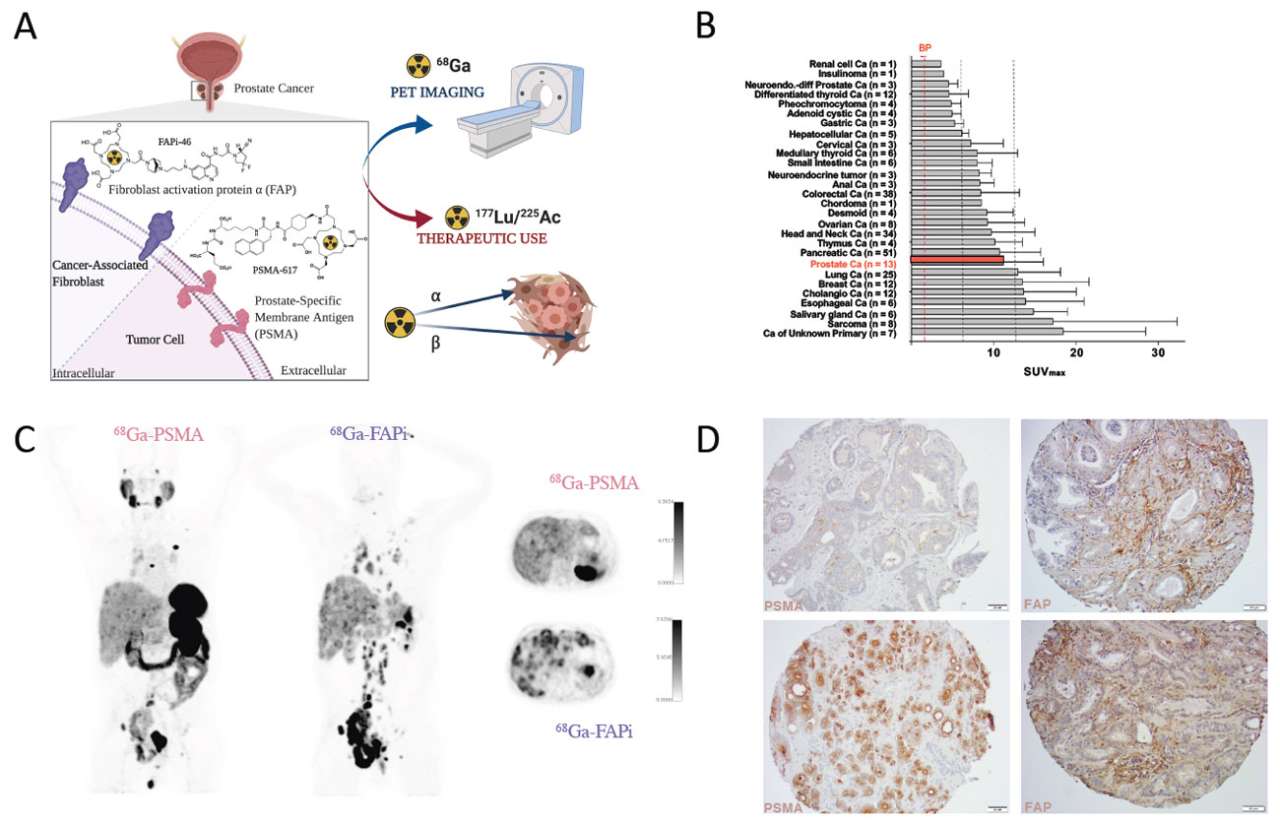
Basic principle of PSMA- and FAP-directed theranostics. (A) Gallium-68 labeled radioligands binding to PSMA (68Ga-PSMA) or FAP (68Ga-FAPi) can be used for PET imaging of PSMA and FAP positive tumors, respectively. Labeling of these radioligands with Lutetium-177 or Actinium-225 allows delivery of ionizing radiation specifically to PSMA and FAP expressing cells (radionuclide therapy). Both, PSMA- and FAP-ligands, bind with very high affinity to their target. (B) 68Ga-FAPi uptake (SUVMAX) in various cancers (mean ± SD). Vertical lines indicate SUVMAX cut-offs to define low (blood pool [BP] < x <6), intermediate (6 ≤ x <12) and high uptake (≥12) (adapted from Kratochwil et al., J Nucl Med. 2019;60(6):801-805). (C) 68Ga-PSMA-11 (left) and 68Ga-FAPi-46 (right) PET/computed tomography (CT) images of a mCRPC patient. Left and middle: 3D maximum intensity projections. Right: fused PET/CT axial view. (D) Representative histological images of a prostate cancer tissue microarray demonstrating PSMA (left) and FAP (right) expression (magnification 20x). Black bars represent 100 μm.
PSMA-targeted Theranostics
RNT targeting the prostate-specific membrane antigen (PSMA; 177Lu-/225Ac-PSMA) is an emerging treatment modality in metastatic prostate cancer. However, approximately half of the patients experience treatment failure despite tumor PSMA expression, and relapse occurs invariably. Using prostate cancer mouse models, we have uncovered various RNT resistance mechanisms including (i) low or heterogeneous PSMA expression resulting in insufficient tumor radiation dose delivery; (ii) suboptimal selection of radioisotopes (alpha vs. beta) for specific disease patterns; (iii) upregulated DNA damage and replication stress responses that can be addressed pharmacologically using clinically viable drugs; and (iv) genomic alterations such as inactivating mutations in the tumor suppressor gene TP53. Recently, we showed that combining RNT and PD-1 blockade can synergistically reduce prostate cancer burden, improve time to progression and survival, and may lead to generation of an immunological anti-tumor memory. Together, our translational research aims at providing solutions for inherent and acquired resistance to PSMA-targeted RNT that will allow clinicians to exploit the full potential of RNT for the benefit of patients with lethal prostate cancer.
Fibroblast activation protein-targeted theranostics: A new approach to modulate tumor-stroma interactions
Fibroblast activation protein (FAP) is a serine protease that is overexpressed on cancer-associated fibroblasts (CAFs), which can constitute >90% of tumor stroma, and sometimes on cancer cells. In malignant diseases, FAP expression has been associated with neoplastic transformation, tumor growth, neo-angiogenesis, metastatic potential, treatment resistance and immunosuppression. Using the new FAP-targeted PET probe 68Ga-FAPi which was developed by our collaborators (Prof. Uwe Haberkorn, University of Heidelberg), favorable dosimetry (i.e., high tumor to healthy organ ratio) was established across 28 different cancers. Our research aims at (i) establishing quantitative radiotherapeutic strategies for FAP-targeted RNT that significantly reduce tumor burden and improve survival in our murine cancer models; and (ii) investigating the alterations in signaling, metabolism and immune status triggered by FAP-targeted RNT with the goal to exploit these alterations by administering synergistically acting combination therapies.
We collaborate closely with Drs. Czernin and Calais in the Nuclear Medicine Clinic to clinically translate FAP-targeted theranostics for the characterization and treatment of difficult to treat malignancies marked by the presence of extensive tumoral and/or stromal FAP expression such as metastatic prostate cancer, pancreatic ductal adenocarcinoma, and sarcoma.

Katharina Lueckerath, PhD
Assistant Adjunct Professor, UCLA Molecular and Medical Pharmacology
Katharina is a biomedical scientist who obtained her PhD at the Institute for Tumor Biology and Experimental Therapy (Georg-Speyer-Haus, Frankfurt a. M., Germany). She served as head of the research group Experimental Oncology in Nuclear Medicine at the University of Würzurg (Germany) before transferring to UCLA. Dr. Lückerath’s research focuses on experimental and translational theranostics. Specifically, she investigates mechanisms underlying failure of radionuclide therapy with the goal to develop rationally chosen, translatable radionuclide therapy-based combination regimens that improve quality of life and survival of cancer patients.

Christine Mona, PhD
Assistant Professor, UCLA Molecular and Medical Pharmacology
Christine received her Ph.D. in Pharmacology in 2016 from Université de Sherbrooke. She joined UCLA in 2016 as a Post-Doctoral Research Associate in Nuclear Medicine in the research group of Professor Johannes Czernin. In early 2020, Dr. Mona was promoted to Adjunct Assistant Professor at the Ahmanson Translational Theranostic Division (ATTD). Dr. Mona is a translational researcher at the edge of Chemistry, Pharmacology and Nuclear Medicine. She is animated by applying modern scientific discoveries to the practice of medicine for the benefit of patients. Her program pioneers research in targeted nuclear medicine in different cancer paradigms and covers a broad field of preclinical research and translation to clinical discoveries.