CAR-T Cell Therapy: This is Your Brain on Living Drugs
Summer 2018
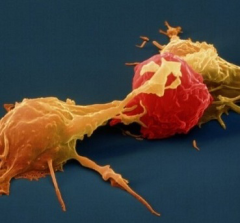
The fields of molecular medicine, cellular therapy and gene delivery have recently advanced to produce the first self-directed immune cell attack for cancer therapy. This is “CAR-T Cell Therapy”. CAR-T stands for chimeric antigen receptor, which is induced in the T cell of the immune system. T cells are taken out of each patient, reprogrammed to attack a feature unique to that patient’s cancer, and administered back to the patient. This approach causes these immune cells to attack the specific cancer cell to each patient. This therapy has been termed “a living drug.”
Developed by UCLA cancer doctors (led by Dr. Arie Belldegrun), this FDA-approved therapy is currently in use against advanced lymphomas and leukemias. As the site of invention of the first FDA approved CAR-T therapy, UCLA Health is a major center for this therapy -- which brings in UCLA Neurology. One of the most concerning side effects of this new treatment is uniquely neurological. Some aspect of the treatment causes the brain to misfire, particularly in understanding language, and also in the possibility of having seizures. It is still unclear what happens to the brain when CAR-T cells are administered to the patient. Some of this dysfunction may be due to the immune molecules, or cytokines, released by CAR-T therapy, although not all of the neurological symptoms can be attributed to cytokine release.
With new therapies come new diseases, and this new disease is termed CRES, or CAR-T related encephalopathy syndrome. Dr. Josh Sasine and Dr. Gary Schiller in the UCLA Bone Marrow Transplant unit, with input from UCLA Neurology, devised diagnosis and treatment guidelines for this new entity. Moving quickly, as this treatment was only FDA-approved in October and the number of patients receiving CAR-T is expanding, these guidelines provide a sure path to follow in a time while the science and patient care are still evolving.
As the Neurologist on service this past summer at Ronald Reagan UCLA Medical Center when the first CAR-T patients were in the hospital, it was clear to this writer that we had a new disease. With close communication, expert understanding and teamwork, UCLA Health developed the rules that allow rapid diagnosis and treatment in a clinical time period of potentially great uncertainty. Guidelines in epilepsy and multiple sclerosis reflect considerable historical experience in these well-recognized diseases. The action with CAR-T therapy shows that the development of treatment guidelines is also rapid and nimble, driven by dedicated experts that work quickly on emerging health events.