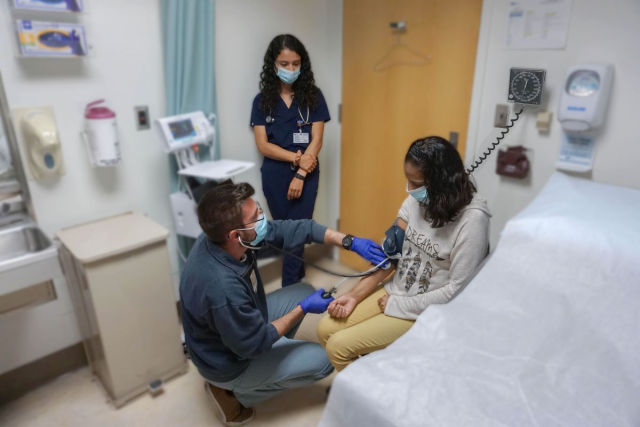
A five-year, $8 million grant from the California Institute for Regenerative Medicine will enable UCLA stem cell scientists to include a wider cross-section of Los Angeles’ diverse population in potentially lifesaving medical research.
In part, the grant will fund the UCLA Alpha Stem Cell Clinic’s continued collaboration with the UCLA Clinical and Translational Science Institute, which supports more than 350 clinical trials at medical centers and community clinics throughout the region.
“Integrating the Alpha Stem Cell Clinic and Clinical and Translational Science Institute enables the university to build on the breadth and depth of investigators, support staff and infrastructure that already exist here,” said Dr. Noah Federman, a UCLA Health professor of pediatrics and orthopaedic surgery, and director of the Alpha Stem Cell Clinic. “Together, we’ll continue to focus on accelerating innovative stem cell and gene therapies to patients while meeting the basic health care needs of underserved communities.”
Established in 2014 with Dr. John Adams as its first director, and under the umbrella of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research, the Alpha clinic provides resources to help UCLA researchers overcome common roadblocks to launching clinical trials and supports their efforts to enroll Californians in need, regardless of their race, gender, age, socioeconomic status or geographic location.
To reach those communities, the Alpha clinic will collaborate with UCLA’s Community Engagement Alliance Against Disparities in COVID-19 program, which aims to reduce health inequities related to the pandemic by providing health education, expanding access to health care and increasing the diversity of participants in clinical trials of new medicines.
The clinic’s first use of the Community Engagement Alliance model will focus on connecting people with sickle cell disease to health care resources and clinical trials of potential treatments for the condition. The program will be spearheaded by Federman; Dr. Arleen Brown, co-director of the Clinical and Translational Science Institute (CTSI); Dr. Keith Norris, co-leader of the CTSI's Community Engagement and Research Program; and Dr. Gary Schiller, a UCLA professor of hematology oncology who is one of the leaders of UCLA’s new sickle cell disease center which opened in Fall 2022.
“There are a variety of different gene therapy approaches for sickle cell disease being developed and tested in clinical trials at UCLA and elsewhere,” Schiller said. “Our goal is to help accelerate those trials, clear the path for more therapies to reach clinical trials and ensure people throughout California can access these innovative treatments.”
The Alpha clinic also will use funds from the new grant to build on UCLA’s existing training programs in cellular and gene therapy as well as regenerative medicine. Those programs provide education and career development to Los Angeles high school students as well as to UCLA undergraduate, graduate and medical students, postdoctoral fellows and junior faculty.
“These programs are designed to engage students who are interested in making a difference through health care at the earliest possible point and support them all the way through their faculty years,” said Federman, who also is a member of the Broad Stem Cell Research Center and of the UCLA Jonsson Comprehensive Cancer Center. “Not only are we building a workforce to deliver therapies to patients in need, but we are also cultivating the next generation of brilliant scientists.”
UCLA’s grant application was unanimously recommended for funding by the state stem cell agency’s scientific grant reviewers, who lauded the proposal as “exceptionally well-planned and executed” and commented that it “will provide significant impact for patients, trial sponsors, health care providers and healthcare students and trainees.”