UCLA Research Alert
FINDINGS
A study by researchers at the UCLA Health Jonsson Comprehensive Cancer Center suggests that injecting engineered dendritic cells directly into cancerous lung tumors can help promote a stronger immune response, causing more T cells to become active and attack the cancer more effectively. When tested in mice with non-small cell lung cancer, the team discovered that combining this therapy with immune checkpoint blockade, a type of immunotherapy, made the treatment even more effective.
BACKGROUND
Immune checkpoint blockade has been revolutionary for treating patients with non-small cell lung cancer. However, the majority of patients with lung cancer do not benefit from the treatment, and many experience disease progression after an initial response. This can occur because the immune system doesn't recognize the tumor as a threat or the environment around the tumor suppresses the immune response. Scientists have found that certain molecules called chemokines, specifically CXCL9 and CXCL10, play a crucial role in attracting immune cells, particularly activated T cells, to the tumor site. When these molecules are present in high amounts, they can help the immune system fight cancer more effectively.
To help enhance the effectiveness of immune checkpoint blockade, the UCLA team explored an approach called in situ vaccination with gene modified dendritic cells, which involves injecting immune-stimulating, chemokine gene-engineered dendritic cells directly into the tumor, which can boost the body's immune response against cancer.
RESULTS
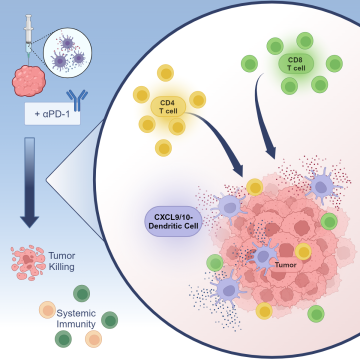
In this study, scientists genetically modified dendritic cells to produce CXCL9 and CXCL10 to help increase T cell infiltration and activation within the tumor. They then injected the modified dendritic cells directly into the tumors in mouse models of non-small cell lung cancer. They found that this approach increased the number and activity of T cells in the tumor and slowed down tumor growth in these models, even in cases where tumors were resistant to standard immunotherapy. Additionally, they observed that this therapy helped to establish a long-lasting immune response against the cancer. Studies analyzing genetic data from lung cancer patients suggest that the CXCL9/10-DC therapy could be particularly beneficial for patients with certain genetic characteristics associated with resistance to standard-of-care immunotherapy.
IMPACT
The results of the UCLA study suggest that using CXCL9 and CXCL10-producing dendritic cells alongside immunotherapy can be a promising strategy to overcome treatment resistance and improve clinical outcomes for patients with non-small cell lung cancer.
JOURNAL
The study was published in Cell Reports Medicine.
AUTHORS
The study’s co-senior authors are Dr. Steven Dubinett, dean of the David Geffen School of Medicine at UCLA, and Dr. Bin Liu, adjunct professor in the division of pulmonary and critical care medicine. Both are members of the UCLA Health Jonsson Comprehensive Cancer Center and Dubinett is a member of the Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research at UCLA. The study’s co-first authors are Dr. Raymond Lim, a former postdoctoral researcher at the David Geffen School of Medicine at UCLA, and Dr. Ramin Salehi-Rad, assistant clinical professor in the division of pulmonary and critical care medicine at UCLA. Other authors, all from UCLA, are Dr. Linh Tran, Dr. Michael Oh, Camelia Dumitras, William Crosson, Dr. Rui Li, Tejas Patel, Samantha Man, Cara Yean, Jensen Abascal, ZiLing Huang, Stephanie Ong and Dr. Kostyantyn Krysan.
FUNDING
The study was funded in part by the Tobacco-Related Disease Research Program predoctoral fellowship award, the Career Development Award-2 from the Department of Veterans Affairs, Biomedical Laboratory Research and Development Service, the National Heart, Lung, and Blood Institute, the UCLA Technology Development Group Innovation Fund and the Merit Review Research Funds from the Department of Veterans Affairs.