UCLA Research Alert
BACKGROUND
One of the hallmarks of cancer cell development is its dependence on sugar, especially glucose, to grow and divide. Scientists have long been studying how to restrict or block this process that promotes tumor growth, called glycolysis, from happening as a possible effective strategy against cancer.
Previously, researchers from the UCLA Health Jonsson Comprehensive Cancer Center identified a specific protein sodium glucose transporter 2, or SGLT2, as a mechanism that lung cancer cells can utilize to obtain glucose. Drugs that inhibit SGLT2 are already FDA approved for other conditions and the UCLA team found these drugs could also delay the development of lung cancer and improved survival when tested in mice, suggesting these drugs could be repurposed for lung cancer treatment.
However, while inhibiting glycolysis can slow down the growth of tumors, the researchers found it can also make cancer cells more aggressive, making the cancer harder to treat. This led the team to look at other mechanisms of resistance in the tumors that still grow while being treated with SGLT2 inhibition that may link glucose restriction to increases aggression.
FINDINGS
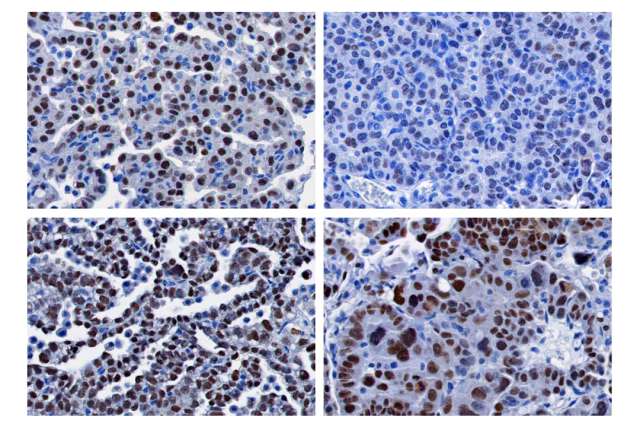
Researchers found that by restricting glucose in lung cancer cells, it caused the cells to lose their specialized features, making them more aggressive. This change was linked to alteration in certain molecules and how they modify DNA structure. One of those molecules was alpha-ketoglutarate, which plays a pivotal role both in energy metabolism and in gene regulation. Reduced levels of this molecule affected how genes are turned on and off, activating HIF1α, a transcription factor known to play a role in making cancer cells more aggressive.
This led the researchers to discover a specific set of genes, controlled by HIF1α, that could predict how aggressive a cancer might be, giving physicians important information that can help guide treatment decisions. The team also found potentials ways to block the tumor from becoming more upset, or greedy, when deprived of its nutrients.
“While we still need to further explore the intricacies of this mechanism, our findings point to a potential therapeutic strategy using a combination of treatments involving epigenic modulators or HIF inhibitors to counter the unintended effects of glucose restriction,” said senior author of the study Dr. Claudio Scafoglio, assistant professor of pulmonary and critical care medicine at UCLA and member of the UCLA Health Jonsson Comprehensive Cancer Center.
IMPACT
This study provides crucial insights into the role of glucose restriction in driving an aggressive phenotype in lung cancer. The discovery suggests a new possible combination approach to treat early-stage lung cancer, using a glucose inhibitor and an epigenetic inhibitor that are already available for other conditions that can help reduce tumor growth and offset aggressive behavior. More work is underway to find the right approach to prevent starvation-induced de-differentiation without causing significant side effects.
JOURNAL
The study was published in the journal Cancer Research, a journal of the American Association for Cancer Research.
AUTHORS
The senior author is Dr. Claudio Scafoglio, assistant professor of pulmonary and critical care medicine at UCLA and a member of the UCLA Health Jonsson Comprehensive Cancer Center. The study’s first author is Pasquale Saggese, a postdoctoral scholar in the Scafoglio Laboratory.
Other UCLA authors include Aparamita Pandey, Martin Alcaraz Jr., Eileen Fung, Abbie Hall, Jane Yanagawa, Erika Rodriguez, Tristan Grogan, Orian Shirihai and Dr. Steven Dubinett.
FUNDING
This work was supported in part by the American Cancer Society and the National Cancer Institute.