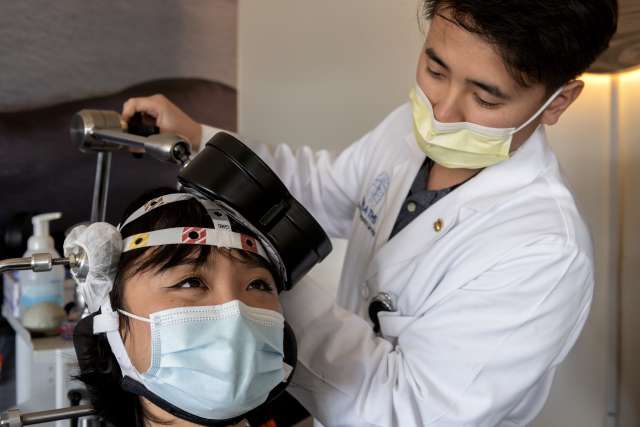
A new study from UCLA Health researchers demonstrates that a novel treatment is effective in most patients with major depressive symptoms even after multiple failed courses of antidepressant medication. The treatment, repetitive transcranial magnetic stimulation (rTMS), may work even more rapidly than past findings have suggested, starting to alleviate symptoms as quickly as one week.
Researchers from the Neuromodulation Division of UCLA’s Semel Institute analyzed the outcomes of hundreds of patients treated at UCLA Health from 2009 to 2022 with rTMS therapy, which uses magnetic fields to effectively “rewire” the brain’s circuitry. The new study published this week in Psychiatry Research found that 54% of patients exhibited clinical response (at least a 50% improvement) in mood symptoms when examined using multiple depression rating scales.
“We have a unique approach to rTMS treatment at UCLA,” said the study’s lead author Dr. Michael K. Leuchter, a senior psychiatry resident at the Jane and Terry Semel Institute for Neuroscience and Human Behavior. “In our ‘precision TMS’ model, patients see a psychiatrist at every treatment and we measure symptoms weekly with multiple rating scales, following a measurement-based care approach.”
This approach allowed UCLA researchers to assess treatment benefit with greater fidelity and accuracy than previous studies using fewer measurement scales.
“What we're seeing in our analysis of our large data set is that a majority of patients get significantly better,” Leuchter said. “What’s most exciting to see is that these patients generally start reporting improvement within a week of starting treatment, even though the treatment itself continues for several weeks to build the full benefit.”
TMS uses magnetic fields to stimulate brain circuits and can target those involved in mood regulation. Patients generally receive 20-30 minute treatment sessions five days per week for a period of six to eight weeks.
Compared to most antidepressant medications, rTMS is relatively new, having been approved by the U.S. Food and Drug Administration in 2008 for treatment of medication-resistant major depressive disorder.
The effectiveness of rTMS has previously been observed as quite variable, with reported response rates ranging from 30-60%. Researchers at UCLA have been working to understand this variability and improve predictions of which patients are most likely to benefit from the therapy.
Leuchter and his colleagues studied the outcomes of 708 patients treated with TMS for a six-week period at UCLA with four widely used depression rating scales. They found that the depression rating scales that doctors use to assess the effectiveness of the treatment could be a large factor contributing to this variability. Across all four scales studied, researchers discovered that 54% of patients reported a significant response on at least one rating scale. If only one scale was used, up to a third of positive responses to treatment could be missed, according to Leuchter.
“Using multiple scales rather than one allows us to better detect and characterize the effectiveness of rTMS treatment for the many different faces of depression,” Leuchter said.
Additionally, early improvements reported within five or 10 treatments were found to be significant predictors of how well a patient will respond throughout the course of treatment. Leuchter said this could help doctors determine whether or when to modify their clinical approach for a patient.
Article: A Comparison of Self- and Observer-Rated Scales for Detecting Clinical improvement During Repetitive Transcranial Stimulation (rTMS) Treatment of Depression Leuchter et al. Psychiatry Research, 2023, 115608, ISSN 0165-1781, https://doi.org/10.1016/j.psychres.2023.115608