Tom Macias is preparing for his brother’s kidney-trans - plant surgery. Every day, for five days, he gives himself multiple shots of a powerful drug that will draw the cells of his bone marrow into his blood. The injections cause bone pain, a deep ache that awakens him in the middle of the night.
But his discomfort is nothing compared with that of his younger brother, Andrew, who is suffering from end-stage kidney disease. Tom has watched helplessly as Andrew’s once-muscular frame withered and became frail under the burden of his illness. Now, it takes several ses - sions a week of dialysis to keep Andrew alive. The treatments sap what little strength he has left, leaving him depleted for the next 24 hours.
So, in addition to accepting the risks of surgery to donate one of his kidneys to his brother, Tom gives himself the shots and grits through the pain. If this works, Tom and Andrew — the first patients in a clinical trial for a new program being developed at UCLA that aims to liberate transplant recipients from the lifelong post-surgical necessity of immunosuppressive drugs — may find themselves at the forefront of a dramatic scientific achievement, the kind that can change the future course of medical care.
After five days of injections, Tom drives to UCLA’s blood and plasma donation center. There, a technician connects him to a machine — a needle in each of his arms, one to draw his blood, now rich with stem cells that have been forced from his bone marrow, and the other to return it to his body after the stem cells have been separated out. After Andrew receives Tom’s kidney in a surgery at Ronald Reagan UCLA Medical Center, those stem cells will be infused into Andrew’s body.
The goal: To achieve “tolerance.” By trans - planting both a donor’s organ and stem cells, the immune system of the recipient is primed to accept the new organ as its own — to recognize it as “self” — without rejection. And without rejec - tion, there is no need for harsh immunosuppres - sive drugs to tamp down the attack the recipient’s body would otherwise launch.
That, says UCLA renal transplant surgeon Jeffrey L. Veale, MD (FEL ’06), “is the Holy Grail of transplant surgery.”
UCLA IS NOT THE FIRST MEDICAL CENTER TO PERFORM THIS PROCEDURE. It is the fourth in the U.S., and the fifth in the world, to do so. But Dr. Veale and his colleagues hope to advance it further than the other centers have. While those centers have successfully performed this protocol on well-matched sibling donor-re - cipient pairs, like Tom and Andrew Macias, as well as with non-sibling pairs, it is Dr. Veale’s goal to extend the procedure to transplants involving deceased donors. That is where the real difference will be made, say Dr. Veale and others involved in the project, noting that deceased donors accounted for more than 77% of the 22,800 kidney transplants that were performed in the United States in 2020.

It is a goal that has a lot of people in the field very excited. “There is an opportunity at UCLA to do something that’s never been done before — to do a deceased-donor transplant using not just the deceased donor’s organ, but also the deceased donor’s stem cells to achieve tolerance,” says Thomas Mone, CEO of the regional organ-dona - tion nonprofit OneLegacy Foundation. “That is immensely more complex than doing this with a living donor, which is complex enough.”
Mone’s colleague, Garet Hil, founder and CEO of the National Kidney Registry, agrees. “It’s a very heavy lift. But UCLA may be able to crack the code to actually achieve it. It takes a lot of courage to try to do something like this,” he says.
If, as its proponents hope, that goal can be achieved with a kidney, it could change trans - plant medicine on a massive scale, making the possibility of a transplant without the tether of lifelong immunosuppressive drugs a reality for recipients of other organs as well — from hearts and lungs and livers to composite-tissue allografts such as arm, leg or face transplants. Reaching that goal “opens up a whole new world,” Dr. Veale says.
Getting there is a long journey, one that already has taken Dr. Veale nearly six years, beginning with a pitch he made to Mone and OneLegacy Foundation to help fund his work. The organization committed close to $2 million to support Dr. Veale’s efforts.
And it takes a multidisciplinary team that engages dozens of experts from across diverse specialties such as nephrology, urology, hematology, radiation oncology and other depart - ments. “It requires a lot of interplay between different divisions,” says transplant nephrologist Erik L. Lum, MD (RES ‘09, ‘10). “This really demonstrates the strength of a place like UCLA. You can’t do this just any - where. It’s a huge collaboration.”
UCLA already is far along on its journey to achieve this ultimate goal. In collaboration with OneLegacy Foundation, the university has devel - oped and applied for a patent for new technology to recover stem cells from a deceased donor. “This is something that no one else has ever done,” Dr. Veale says. Though he is constrained from describing the procedure in detail, Dr. Veale says that university transplant surgeons hope to be able to employ this new process within the coming year, after it goes through all necessary reviews and approvals.
THE SCIENCE LEADING UP TO THE DEVELOPMENT OF THE TRANSPLANT-TOLERANCE APPROACH HAS BEEN EVOLVING FOR DECADES. Samuel Strober, MD, professor of immunology and rheuma - tology at Stanford University, has de - voted his career to studying the science that could lead to transplant tolerance. He has, since the 1980s, focused on the process known as “mixed chimerism” — the blending of a donor’s and re - cipient’s immune systems through an infusion of the organ donor’s stem cells shortly after transplantation to prompt the recipient’s body to recognize, rather than reject, the new organ.
Solid-organ transplants have been successfully performed since the 1950s, but they have always required a lifelong regimen of powerful medications to prevent the recipient’s immune system from identifying the new organ as a foreign invader and attacking it. But these immunosuppressive drugs carry with them a number of serious potential complications, including increased risk of cancer, infection, diabetes, hypertension and heart dis - ease. In the case of kidney transplants, immunosuppresive therapy carries an unfortunate irony; because these powerful medications are filtered through the kidneys, they eventually overwhelm and overtax the new organ that they are prescribed to protect. Even with the best drugs, about half of kidney transplants still are lost to chronic rejection in about 15 years, Dr. Strober says. For the patient, that means having to go back on dialysis or undergo a second — perhaps even a third — transplant.
The goal of the tolerance protocol is to extend the survival of the transplant - ed kidney by encouraging the immune system of the host to live in harmony with the new organ. The hope, Dr. Veale says, is “one kidney for life.”
Dr. Strober began exploring the possibilities of transplant tolerance with animal studies in the 1960s, eventually identifying the elements that have led to dramatic successes for Stanford’s program, where patients who have undergone the transplant-tolerance protocol have survived without immunosuppression for 15 years.
“Because I have worked so long on it, I am highly motivated and very interested in seeing this approach expanded to large numbers of patients,” Dr. Strober says. “I view what we’re doing with the matched patients as a step toward working with a much larger pool of patients, the mismatched pairs.”
Dr. Veale wants to take that baton and run with it. He and Dr. Lum — who as a fellow at Stanford was a member of the team performing the first transplant-tolerance procedures — have worked closely with Dr. Strober, and with surgeons from Stanford, to design UCLA’s new approach.
Just as the transplant-tolerance protocol blends the immune systems of two distinct individuals, it also blends scientific disciplines that don’t ordinarily overlap. “Stem-cell trans - plants and solid-organ transplants are usually conducted independently of each other,” says bone marrow-transplant specialist Neil Kogut, MD, who was nearing retirement after a long career overseeing the bone-marrow-transplant program at Kaiser Permanente when he was re - cruited to work with Dr. Veale on UCLA Health’s transplant-tolerance protocol. “These are very separate worlds that the doctors and researchers in these fields occupy. This protocol is a unique opportunity to bring stem-cell transplantation and solid-organ transplantation together to try to achieve something very positive for transplant recipients. It is a unique synergy.”
ON THE DAY IN MARCH OF ANDREW’S TRANSPLANT SURGERY, THE BROTHERS AND THEIR WIVES ARRIVE BEFORE DAWN at Ronald Reagan UCLA Medical Center. Tom and Lettie had been engaged for nearly two decades before they wed just weeks before the surgery. “It was just because of everything that was going down,” Tom says. “And, also, for Lettie to be able to be here with me.”
Andrew and his wife, Deanna, have been mar - ried since 2017. He was already sick with kidney disease when they met, and he dreams of a day when she can see him as fit and healthy as he was in his football-playing days.
Lettie and Deanna were both at the family Christmas party in 2019 when Tom gave Andrew a small, red box with a unique gift inside. A few weeks earlier, Tom had learned he was a perfect match to donate a kidney to his younger brother. But he hadn’t told anyone other than Lettie. Andrew shook the box, commenting on how light it felt. “Probably something from the 99 Cents Store,” he joked.
Inside, he found a note. “My dearest brother,” it read. “I found out I am a perfect match. I am giving you the gift of life.” “Everybody started crying,” Tom says. “Andy was choking up as he was reading.” Now, more than a year later, they are ready for surgery.
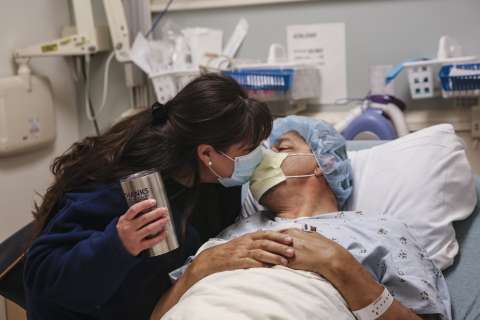
Tom is rolled on a gurney into Operating Room 19, on the second floor of the hospital, where H. Albin Gritsch, MD (RES ‘91), John Jergens Chair in Kidney Transplantation and surgical director of UCLA’s kidney-transplant program, awaits. Dr. Gritsch and his team will remove Tom’s left kidney, which they will then bring to an adjacent operating room to be transplanted into his brother.
Working through four small incisions, Dr. Gritsch inserts a small camera and instru - ments to perform the procedure. The image from inside Tom’s body is displayed on a large screen. Maneuvering within the confines of the abdominal cavity, Dr. Gritsch points out other organs to his residents as one moves them aside to access the kidney. “It’s always surprising how tightly packed the organs really are, after you see them spaced out in illustrations,” he says. “Like a Rubik’s Cube.”
As the team works, nurses fill a tub with saline and ice to receive Tom’s kidney. While it still is inside Tom’s body, the surgeons sheathe the kidney in plastic and then pull the organ out through a 10 cm cut. As Tom’s kidney rests in its chilly bath, the surgeon cuts away the plastic and inserts an IV with saline to flush away the blood. The organ, about the size of a mango, turns a pale tan.
Next door, in OR-18, Dr. Veale turns off the house music he has been listening to. Andrew is already prepped, an incision in his torso and his own kidney removed. The new kidney arrives, and Dr. Veale lifts it from the tub. “This is a good kidney,” Dr. Veale says, with obvious delight. “It’s big. Lotta horsepower with this kidney.”
He trims some fat from the organ, fits it in place, and he and another surgeon begin suturing. Once the new organ’s veins and arteries are connected, Dr. Veale removes the clamps and blood streams through, turning the kidney a deep pink. Within seconds, he confirms that the kidney is making urine. Andrew will no longer need dialysis.
In a nearby room, Lettie and Deanne look up from their phones when Dr. Veale comes in. “It went really well,” he tells them. “The kidney pinked up nicely, and it made urine right away.” Now he has to tell the other members of the transplant-tolerance team that it all went well. “I have dozens of emails to send,” he says.
Tom is discharged from the hospital the next day; Andrew will spend several more days recovering. Before leaving the hospital, Tom stops by his brother’s room. It is the first time, he says, that he has really seen how sick Andrew was.
“He’s my younger brother, and he looked 15, 20 years older than me,” Tom says. “He looked frail as can be. I saw death on his face.” Now, perhaps, there is hope that Andrew can be healthy again.
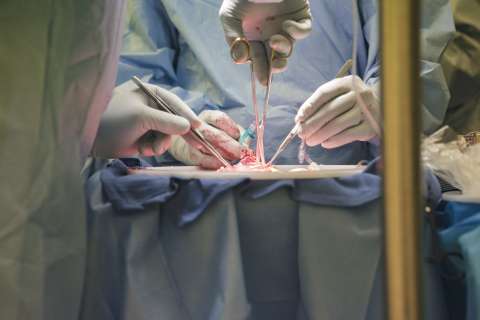
THE SURGERY IS OVER, BUT ANDREW’S JOURNEY IS FAR FROM FINISHED. The day after the operation, Andrew begins a series of treatments called “total lymphoid irradiation” to kill off enough of his own stem cells to make room for the infusion of Tom’s. Once used to treat Hodgkin’s disease, the procedure now is employed primarily with post-transplant patients to help prevent graft-versus-host disease.
Andrew began meeting with radiation oncologist Ann Raldow, MD, MPH, weeks before his surgery. “Patients come in before their transplant, and we develop a radiation-therapy plan that is specific to their anatomy,” taking care to avoid exposing the newly transplanted kidney to radiation, Dr. Raldow explains.
As part of his preparation, Andrew had nine tiny dots — radiation tattoos — inked onto his upper and lower chest, lower abdomen and pelvis, sides and shoulders. These will serve as guides to help the treating therapist align his body precise - ly during the procedure.
When Andrew arrives for the treatment, he is led to a room with a high-tech machine in the center. Andrew is weak, and he moves slowly. “I can feel things changing in my body,” he says
A nurse helps him onto the machine’s platform, and two therapists strap his body in place. They align his torso using lasers and the tattoos as their guide. After technicians cover his body with a sheet and his face with a netting to keep his head and neck in position — the radiation beams begin just below Andrew’s neck — the treatment begins.
The procedure is painless. “It’s like getting a very targeted X-ray,” Dr. Raldow says.
Music from the ’70s rock band Bread plays in the treatment room. “His choice,” a tech says. Andrew is motionless in the machine as the techs close the heavy vault-like door to the room. The words “Beam in use” light up above the door and the machine begins rotating around Andrew’s body, circling his torso and moving lengthwise from his neck to his feet. In an adjacent room, doctors and nurses monitor a 3D rendering of the procedure on a computer screen.
Andrew will receive a total of 10 radiation treatments. Once that is complete, his immune system will be primed to receive the infusion of his brother’s stem cells.
Before that can take place, however, Tom’s stem cells must be processed to create a cocktail of his stem cells and T cells for the infusion. That work was done in the laboratory of Donald B. Kohn, MD, Distinguished Professor of Microbiology, Immunology and Molecular Genetics and of pediatric hematology-oncol - ogy. Over the course of his more than 30-year research career, Dr. Kohn has studied the therapeutic applications of blood stem cells. The opportunity to help develop UCLA’s trans - plant-tolerance protocol was tantalizing. “I’ve always thought this is exactly the direction in which transplantation needs to be going,” Dr. Kohn says. “If we can help guide the immune system to not reject the graft, as this does, that is a significant step forward. From here, we need to learn how to do this with heart transplants and liver transplants, and all the other transplants, to avoid the complications of immune suppression for our patients.”

When the day of the infusion comes, Deanna is by Andrew’s side as his brother’s stem cells are dripped into his vein. “The stem cells smell like garlic mixed with corn,” she recalls. “The nurse said he would be able to taste it, so they brought him some lollipops to suck on.”
It will take time to know how successful the procedure proves to be. If full mixed chimerism is achieved — if, as Dr. Lum explains, “a significant portion of Andrew’s blood and bone marrow are part Tom and part Andy” — and is sustainable, then Andrew’s new kidney will thrive without the need for immunosuppressive drugs.
THE SURGERY, RADIATION AND STEM-CELL INFUSION TOOK A LOT OUT OF HIM, and as Andrew recovers at home, he feels weak and exhausted. Between tracking his blood pressure and taking his pills, he’s on a strict schedule, and some days it takes all his energy just to keep up with it. He regularly has his blood drawn so his medical team can monitor his progress.
“It’s not for the faint of heart, that’s for sure,” Andrew says. “But if this is the price I have to pay to have a kidney that I can keep for the rest of my life, I’m willing to do this 20 times over. Overall, I’m happy,” he continues. “I just can’t wait to get to where I can do things, like go back to work and work on my yard and drive my muscle car around.”
Weeks pass. Andrew still feels frail, but he can tell he’s slowly getting stronger. And the doctors have good news: His blood tests show that chime - rism is forming. Dr. Lum is cautiously optimistic. “It’s scary, but in a good way,” he says.
As Andrew continues to heal, he reflects on the profundity of the experience. He marvels at the support Deanna has provided throughout this journey, the way her parents have extended themselves and how his friends have kept up their presence in his life.
And he thinks about his brother, Tom, who gave a portion of himself so that Andrew could be healthy again. “There’s no words,” Andrew says, his voice cracking. “There’s no words that I can say that would live up to what he’s done for me. I’m a grandpa, and I want to be here for my grandkids and for my children and for my wife.”
Several more weeks pass, and on a classically sunny L.A. weekend, Andrew and Tom and their wives are enjoying a party with OneLegacy Foundation staff and supporters and more than two dozen UCLA Health doctors, nurses, coordinators and other clinical workers who have been involved in the transplant-tolerance procedure. Andrew looks healthier than he has in years. Doctors have taken him off of all but one immu - nosuppressive drug, and he looks forward to the day when he will be free of that, as well.

As the partygoers celebrate, a karaoke machine in the corner of the stage catches Andrew’s eye. He steps up, grabs the microphone and lets loose with Bon Jovi’s “Livin’ on a Prayer”:
Oh, we’ve got to hold on, ready or not You live for the fight when it’s all that you’ve got Woah, we’re half-way there Woah, livin’ on a prayer Take my hand, we’ll make it I swear Woah, livin’ on a prayer