About the Clinical Trials Unit
The UCLA AIDS Prevention and Treatment Clinical Trials Unit
The UCLA AIDS Prevention and Treatment Clinical Trials Unit (UCLA APT CTU), housed within the UCLA CARE Center, strives to make significant contributions to the development and conduct of HIV treatment and prevention studies through DAIDS-sponsored HIV Clinical Research Networks. The UCLA APT CTU originated with two sites in Los Angeles in 1986 and has evolved into a highly functional, multidisciplinary clinical research unit that includes five Clinical Research Sites (CRS) that contribute to clinical trials under the purview of three HIV treatment and prevention networks.
The investigators at each of these CRSs have made significant contributions to the development of effective antiretroviral therapy (ART) for diverse patient populations, as well as both biomedical and behavioral interventions for the prevention of perinatal, sexual, and drug use-related transmission of HIV/AIDS over the past thirty-five years. Despite these past accomplishments, significant challenges remain:
- Reducing the numbers of new infections among key populations;
- Achieving viral suppression among greater numbers of people;
- Reducing the burden of tuberculosis globally; and
- Reducing morbidity from chronic diseases and hepatitis B virus among people living with HIV.
The UCLA APT CTU is poised to make important contributions to evaluating interventions through design, leadership, and participation in clinical trials that address these challenges.
UCLA AIDS Prevention and Treatment Clinical Trials Unit Structure
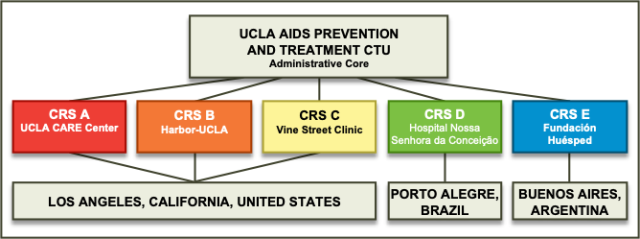
The Clinical Research Sites
UCLA CARE CRS
The UCLA Center for Clinical AIDS Research & Education (CARE), is administratively housed in the Division of Infectious Diseases, Department of Medicine and is a core-funded site for the ACTG and HPTN. The CARE CRS is located four miles from the UCLA campus in West Los Angeles adjacent to the areas where the highest rates of HIV infection are reported among MSM. The site provides primary HIV care and HIV prevention clinical services and has an extensive track record in the conduct of DAIDS network sponsored research in the areas of treatment and prevention. Click to see a list of clinical trials currently enrolling at this site.
CRS Leader: Raphael J. Landovitz, MD, MSc
Email address: [email protected]
UCLA Vine Street CRS
The UCLA Vine Street Clinic is administratively under the Department of Family Medicine at UCLA, is a DAIDS core-funded CRS for the HPTN. Located in a storefront in Hollywood, an area with the highest number of new HIV infections in Los Angeles County, the site has the capacity to conduct clinical research protocols on behavioral and biomedical HIV prevention (having successfully participated in both AMP and HPTN 083), behavioral and biomedical therapies for drug dependence and related basic science protocols. The site also has certifications to provide some basic care and treatment services under the auspices of UCLA medical services.
CRS Leader: Steve Shoptaw, PhD
Email address: [email protected]
Harbor-UCLA CRS
Located in Torrance, California, in the southwest of LAC, Harbor-UCLA Medical Center is an affiliated teaching hospital for the David Geffen School of Medicine at UCLA. The medical center maintains and staffs an HIV clinic at the medical center itself and another in Long Beach. The Division of HIV Medicine at Harbor-UCLA is an academic research program that provides primary outpatient care and inpatient consultative services for those with HIV infection. The Harbor-UCLA CRS has been a core-funded CRS participating in the ACTG for nearly 20 years. The CRS is located on the grounds of Harbor-UCLA Medical Center in an area reporting the second highest rates of HIV in the LAC. The site provides both patient care and conducts a wide range of research on HIV-infected adults. The CRS largely derives its study participants from a broad and diverse population of patients receiving HIV-related services through its publicly funded HIV programs.
CRS Leader: Eric Daar, MD
Email address: [email protected]
Conceição, Porto Alegre, Brazil
Hospital Nossa Senhora da Conceição has a catchment area that covers approximately one third of the city of Porto Alegre’s 1.5 million inhabitants, providing health care to the most disenfranchised populations in the metropolitan area, where major health problems coexist. Conceição is also the largest publicly funded general hospital in the South of Brazil and is the tertiary referral center for all basic health units within its catchment area. Conceição has been a DAIDS core-funded CRS since 2000, has contributed a significant number of participants to pediatric and adult treatment trials, HIV-infected pregnant women and their infants to perinatal and PK trials, and MSM, heterosexual couples, and drug-using participants to HIV prevention studies.
CRS Leader: Breno Santos, MD
Email address: [email protected]
Fundación Huésped, Buenos Aires, Argentina
Fundación Huésped is a Non-Governmental Organization with clinical research, HIV and HCV therapeutics, and HIV prevention as its core functions. Fundación Huésped provides direct services free of charge to people affected by or at risk for HIV, including HIV testing, ART, STI testing, HIV prevention services (including PrEP), mental health, legal and social support. Fundación Huésped is the premiere clinical research agency dedicated to HIV in Argentina. Previously an NICHD-funded IMPAACT site, Fundación Huésped currently is a protocol-specific site for HPTN 083 and has been selected as a protocol-specific site for the upcoming HVTN 706.
CRS Leader: Pedro Cahn, MD, PhD
Email address: [email protected]