Brain Tumor Drug Gets a Second Look
Combining Multiple Types of Imaging in the Brain can Reveal Whether the Drug is Working
May 29, 2020: FOR IMMEDIATE RELEASE
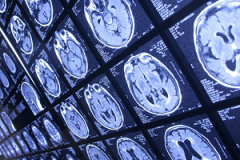
By combining three types of advanced imaging techniques, researchers were able to predict which patients would respond to a drug targeting the deadly brain tumor glioblastoma multiforme. The technique will help researchers conducting phase 2 trials see how well the drug is performing, and identify patients who are responding.
“We’ve never really had this opportunity before to noninvasively evaluate this type of biological effect,” said Dr. Timothy Cloughesy, MD, a professor of neuro-oncology at the David Geffen School of Medicine at UCLA and one of the study’s senior authors. The ability to see the effect the drug is having on the tumor, Dr. Cloughesy said, allows researchers to establish dosage guidelines and select patients with tumors that are likely to respond to the drug.

Glioblastoma multiforme (GBM) is hard to treat because most drugs can’t cross the blood-brain barrier to reach the tumor in the brain. Half of patients diagnosed with GBM die of the disease within the 15 months, and 95% die within 5 years.
In 2012, Genentech began testing a drug called GDC-0084 that can be taken by mouth, penetrates the brain, and blocks a key biochemical pathway that the tumor cells need. Genentech ultimately declined to develop the drug any farther, but now it’s getting a second look in the hands of Kazia Therapeutics, an Australian company focused on cancer drugs.
To observe how a brain tumor responds to the drug, researchers rely on non-invasive imaging tools, like MRI. “Unlike other types of cancer, where you can biopsy repeatedly to see if the drug is having an effect, we don’t have that luxury in brain tumors,” explained Benjamin Ellingson, PhD, director of the UCLA Brain Tumor Imaging Laboratory at UCLA, and first author on the study.
The researchers tested different imaging techniques that measure different tumor characteristics to see whether they could observe the effects of the medication on the tumor. They looked at three main characteristics: how much sugar the tumor cells burned for energy, how many new blood vessels the tumor was growing, and the overall size of the tumor.
“We wanted to know, if we applied some advanced imaging, whether or not we could see some early signal of efficacy,” said Dr. Ellingson.
By injecting the patient with sugar labeled with a radioactive tracing atom, then scanning the brain for signs of radioactivity, they could see how fast the tumor is taking up sugar. A decrease in the amount of sugar used by the tumor can indicate that the therapy is working.
Second, they use detailed MRI techniques to identify the distinctive blood vessels of the tumor. Tumor blood vessels grow fast and tend to leak, and these techniques reveal how dense the blood vessels are and how much they leak, thus revealing the extent of the tumor.
Another type of imaging reveals how tightly packed the cells are by following how water diffuses in the brain. In healthy brain tissue, water movement follows certain pathways called axon bundles, tightly bound groups of tendrils extending from neighboring brain cells. By looking for water that diffuses away from these defined paths, it’s possible to measure the tumor bulk and see whether it’s growing or shrinking.
Each of these measures is useful, but not all of them are equally informative in all patients. That’s why the team developed a mathematical formula to combine the measurements from all these imaging methods. The result is a metric that clinicians can use in future clinical trials to determine how well the patient’s tumor is responding to the drug.
In December, Kazia Therapeutics announced that GDC-0084 will be included in a high-profile international clinical trial called GBM AGILE, led by Dr. Cloughesy. The GBM AGILE trial is a multi drug, adaptive phase II/III trial designed to speed up the process of testing new therapies. Unlike conventional trials, which test one drug at a time, researchers conducting an adaptive trial can drop medications that aren’t working, or add new ones as they become available. The trial began enrolling patients in August 2019, and will include multiple sites around the United States and worldwide.
The imaging study was published in the journal Clinical Cancer Research. The study’s other authors include Jingwen Yao, Catalina Raymond, David Nathanson, and Ararat Chakhoyan of UCLA, along with researchers at Kazia Therapeutics; Genentech; the University of Texas MD Anderson Cancer Center; Massachusetts General Hospital; and Dana-Farber Cancer Institute.
Media Contact
Marrecca Fiore
310-562-4161
[email protected]