Chair's Message Archive - 2023
December 2023 - Education Update
Fostering Education is a cornerstone of our Department mission. We are pleased to celebrate Trainee achievements and academic success! I would like to highlight some of the accomplishments of our Residents this past quarter.
Congratulations to those Residents who have presented posters or given oral presentations at national meetings!
Poster Presentations
- Dr. Mahmuod Abdeljaber (PGY1)
- The American Society of Dermatopathology 2023 Annual Meeting
- Is This A Drug Rash? Look Again. Cutaneous Myeloid Sarcoma in a Patient Without Known Leukemia: A Diagnostic Challenge
- The American Society of Dermatopathology 2023 Annual Meeting
- Dr. Cullen Lilley (PGY1)
- The American Society of Dermatopathology 2023 Annual Meeting
- Suspected Protracted Calciphylaxis in a Patient with Progressive Lower Extremity Pain
- American Society for Clinical Pathology (ASCP) 2023 Annual Meeting
- Assessing the Impact of Race, Sexual Orientation, and Gender Identity on USMLE Style Questions: Preliminary Results of a Randomized Controlled Trial
- The Informative & Virtuous Role of Autopsy Observation Experiences in Medical Student Education
- College of American Pathologists (CAP) Annual Meeting 2023
- Cutaneous Squamous Cell Carcinoma with Metastasis to the Parotid: A Single-Center Retrospective Histologic Observational Study
- Dr. Ruoji Zhou (PGY1)
- The American Society of Dermatopathology 2023 Annual Meeting
- Cutaneous Carcinosarcoma, a True Biphasic Tumor or a Phenomenon of Divergent Differentiation? – A Rare Case Report
Oral Presentations
- Dr. Mahmuod Abdeljaber (PGY1)
- National Association of Medical Examiners 2023 Annual Meeting
- Stepping into Danger: Exploring Risk Factors for Older Pedestrians
- National Association of Medical Examiners 2023 Annual Meeting
- Dr. Precious Fortes (PGY4)
- International Federation of Placenta Association Meeting 2023
- Does Amsterdam Criteria Applied to Term Placentas with Favorable Fetal Outcomes Show Significant Maternal Clinico-pathologic correlation? An Updated Analysis
- College of American Pathologists (CAP) Annual Meeting 2023
- Diffuse Large B-Cell Lymphoma with Follicular Lymphoma Grade 3B of the Ovary Mimicking a Solid Ovarian Neoplasm
- American Society of Cytopathology Meeting 2023
- Cervical Cancer: A Contemporary Look at an Old Tale
- International Federation of Placenta Association Meeting 2023
- Dr. Joshua Pierce (PGY3)
- Society for Pediatric Pathology 2023 Fall Meeting
- Pediatric Angiosarcoma Masquerading as a Lymphatic Malformation in an Autopsy Case: A Rare Case Providing Insight Into Possible Etiologies for Disease Natural History
- Society for Pediatric Pathology 2023 Fall Meeting
Publications
- Dr. Cullen Lilley (PGY1)
- Dr. Joshua Pierce (PGY3)
Fellowships
I also wish to congratulate our Residents who have recently accepted fellowship offers:
- Dr. Erica Fermon – Transfusion Medicine – Cedars Sinai
- Dr. Alex Oliveira-Kowaleski – Cytopathology – UCLA
- Dr. Joshua Pierce – Dermatopathology – UCLA
- Dr. Amanda Zand – Gastrointestinal/Liver Pathology – UCLA
Congratulations to all on your achievements this past quarter!
Non-ACGME Fellowship Recruitment Update
For the second year, the UCLA Department of Pathology participated in the Association of Pathology Chairs (APC) Unified Approach to fellowship recruiting. The Unified Approach was designed by the APC to help standardize non-ACGME fellowship recruitment timelines in order to give residents more time to choose a subspecialty and interview at multiple programs before being asked to commit to one. The programs participating in the Unified Approach agree to adhere to a common timeline for interviewing and to make offers for their fellowship positions on a specific match day and time. Once the offers go out, the residents have 24 hours to accept the offer before the program can offer the position to another applicant.
This year UCLA offered three positions (2 for General Surgical Pathology and 1 for Women’s Health) for the 2025-26 academic year through the Unified Approach, and we are pleased to say that we were one of the few programs nationally to fill all our listed positions on the first round! The other subspecialty non-ACGME surgical pathology fellowships are not participating in the Unified Approach this year, so we are in the midst of helping the subspecialty division chiefs recruit for those fellows.
November 2023 - Research Cores
The Department of Pathology's Research Service Core laboratories provide expert faculty leadership and innovative instrumentation, technologies and specialized services, to a large number of our UCLA biomedical researchers. Our Cores provide a number of services essential for modern research, including whole genome sequencing, state-of-the-art digital imaging/automated imaging analysis, biobanking, chemistry, hematology, coagulation and endocrine testing for clinical trial work and immune assessment services for clinical trials. I will be highlighting below updates for The Technology Center for Genomics & Bioinformatics (TCGB) and The Translational Pathology Core Laboratory (TPCL).
Technology Center for Genomics & Bioinformatics (TCGB) Update
- In 2023, the TCGB introduced 4 new technologies, 11 new services and offered 23 seminars & trainings in high-throughput sequencing, single cell multiomics and spatial genomics:
- Novaseq X plus
- DNBseq T7
- BD Rhapsody HT Express
- CosMx SM
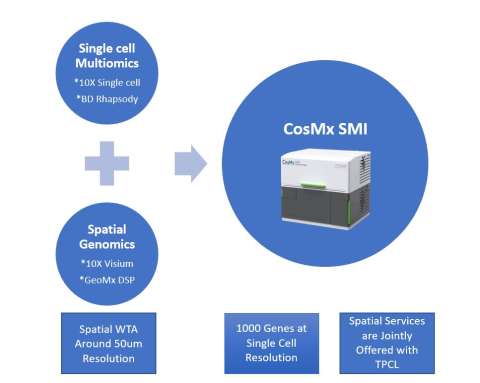
- The TCGB provides multi-dimensional support resulted in substantial scientific, financial and intellectual impact. In the past 5 years, the TCGB contributed to UCLA Health Jonsson Comprehensive Cancer Center (JCCC) accomplishments:
- 166 publications
- 193 awarded grants totaling ~$277M
- 21 patent applications
- The TCGB will continuously make impactful contributions to UCLA investigators' research by developing advanced technologies, services and solutions. In 2024, the TCGB will launch:
- Stereoseq spatial technology & services for whole genome multiomics analysis at a single cell resolution
- Multiomics dataset integration and analysis
- Monthly office hours for Q&A and consultation
The TCGB Team
Top Row: Andrew Rao, YooJin Lee, Lynda Jauregui, Reyna Mathai, JaeMo Kim, Gexin Zhao, Yu-Chyuan Trent Su
Middle Row: Varuzhan Balasanyan, Jo Bodhaine, Lilly Boiton, Melody Wu, Mark I. Duhon, Jahan R. Bruce
Bottom Row: Toni Mckenzie-Mendiola, Denise Llera, Lea DeMarco, Xinmin Li, Hyun-Ju Lim, Catherine Sutarjo, Chao Niu

Translational Pathology Core Laboratory (TPCL) Update
The Translational Pathology Core Laboratory (TPCL), a UCLA Health Jonsson Comprehensive Cancer Center (JCCC) Shared Resource since 1998, provides pathology services critical for basic, translational, clinical, and population cancer research. TPCL provides a College of American Pathologists accredited human tissue biobank, histology (animal/human; frozen/formalin-fixed paraffin embedded), laser capture microdissection, immunohistochemistry, and in-situ hybridization services (established/new antibody optimization), and pathology consultation services. TPCL connects cancer researchers with subspecialty pathologists and was an early adopter of digital pathology, assisting with custom digital image analysis algorithms for complex evaluations of tissue sections.
TPCL provides essential services including the utilization of spatial transcriptomics (see figure). TPCL adapted the spatial gene expression platform Visium (10X Genomics along with supporting the GeoMx (Nanostring) platform (with TCGB). TPCL has also expanded processes to identify and retrieve remnant biofluids with increased customized clinical variables, expanded research blood draw order capabilities within routine clinical lab orders, and added biofluid acquisition, processing, and storage.
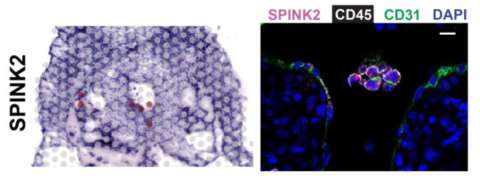
Spatial Transcriptomics (Visium, 10X Genomics) Mapping Human Hematopoietic Stem Cells From Hemogenic Endothelium to Birth
Spatial transcriptomics (Visium) Performed at TPCL in collaboration with Pathology's Technology Center for Genomics & Bioinformatics (TCGB) demonstrated, for the first time generation of hematopoetic stem cells (HSC)s from hemogenic endothelial cells (highlighted by SPINK2) in the aorta of 5 week old embryos. Read More >
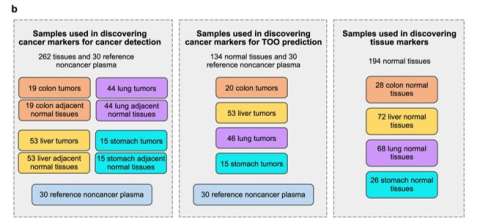
Methylome Sequencing of Cell-Free DNA for Cancer Screening from Patient Plasma Dr. Jasmine Zhou's Laboratory
TPCL provided colon, liver, lung and stomach cancer specimens for assay development. Blood samples were also provided. This allowed for the development of a highly sensitive early cancer screen by Dr. Jasmine Zhou and her team.
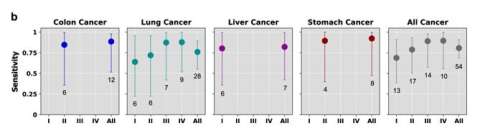
Assay demonstrates high sensitivity for carcinoma, including early stage. Read More >
Additional Updates
Exciting future TPCL directions to support and benefit JCCC research include:
- Automated IF/combined IF/RNA-ISH staining and expansion of RNA (F)ISH services to include miRNA detection;
- Increased clinical variable searches for remnant biofluids;
- Facilitated use of research blood draw requests by non-clinicians;
- Expansion of clinical annotations of biorepository management system to include synoptic reports;
- Upgrade and expansion of image analysis software (Visiopharm);
- Expansion of GSR collaboration to include CosMx (Nanostring) and Stereo-seq platforms; and
- Adding a dedicated staff member to assist with consenting/procurement for special JCCC supported studies
TPCL Team
Top Row: Sam French, Sepehr Hamidi, Joseph Bautista
Middle Row: Rick Huang, Roxana Gonzalez, Jo Anne Olivas, Clara Magyar
Bottom Row: Yunfeng Li, Ko Kiehle, Kim Flores, Chris Creencia, Kody Lopez Devoux (not pictured)

October 2023 - Division Updates
ANATOMIC PATHOLOGY
Stanford Warehouse Project
Earlier this year, the Surgical Pathology Reporting Office (SPRO) completed a major effort to evaluate the materials the Department has been storing offsite, to ensure we are storing essential materials under proper conditions. In addition to old paper files that were long past the date that we were required to keep them, SPRO unexpectedly encountered several surprises. This included blocks irreparably damaged in the distant past by fire, water and mold (see photos below) and many broken slides, which led to further work with the Translational Pathology Core Laboratory (TPCL) to evaluate slide and block research requests. As a result of these efforts, we decided to dispose of our oldest slides and blocks. Overcoming complications from the pandemic and supply chain delays, the SPRO team and collaborators were able to complete the project in just over two years.
The SPRO team packaged 5,882 boxes onto 194 pallets for disposal, including 76.5 tons of old blocks and slides. They were able to recycle many of the old slide cabinets and have been using the recycled cabinets in Santa Monica and Westwood for the past year. They estimate there will be enough recycled cabinets to accommodate our storage for the next five years.
Total Department savings over the next five years from this project are just under $500,000. This includes savings from reduced storage costs and use of recycled cabinets obviating the need to purchase new cabinets.
As is true of many projects, there were many people who contributed to this success. In addition to the SPRO team, Joseph Bautista (TPCL), Rick Huang (TPCL), Dr. Clara Magyar (TPCL), SMH Pathology Staff, the Pathology Facilities team, the Lab Business Office team, Abran Herrera (and the UCLA Stanford Warehouse Team), CHS A Level Loading Dock Team, Dobbie Walton from UCLA EHS and several non-UCLA teams (the CPM Warehouse Team, the Stericycle Team, Modular Data Services and Santa Monica Waste Management) were essential to the success of this project.
Thank you Kelly Bartlone (Manager, Surgical Pathology Reporting Office, Transcription and Referral Lab Sendouts) and SPRO to championing this project and thank you to all who contributed for your hard work and dedication to this effort!



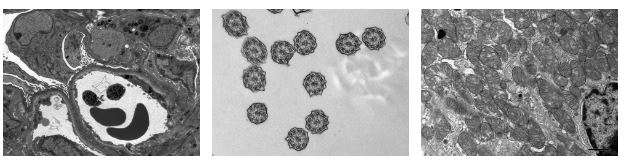
Electron Microscopy Lab
The UCLA Electron Microscopy (EM) lab recently underwent some upgrades and is equipped with two state of the art modern electron microscopes. It serves both the clinical and the academic research community at UCLA and several pathology groups in the region. The team’s activity covers all areas of Surgical Pathology with special expertise in Renal Pathology, Neuropathology, and Cardiac Pathology.

Laboratory Medicine
Clincal Chemistry
- On April 5, the Clinical Chemistry Team has switched to a high sensitivity troponin test for the UCLA health system by working closely with cardiologists, Emergency Department physicians, and hospital leadership. The implementation went seamlessly and successfully. After the implementation, they have identified particular needs and worked with Emergency Department and IT to build additional test codes to address:
- Three high-sensitivity troponin test panels for Emergency Department patients, in-patients, and patients post cardiac interventional procedure, respectively.
- Total Laboratory Automation (TLA): Santa Monica and BURL labs have active TLA. The changes required for the TLA installation at Ronald Reagan Hospital have been more complex, and the lab looks forward to beginning the installation of a new TLA line in the 2024 calendar year.
- The team has validated 34 blood gas analyzers for ICUs and other units throughout Ronald Reagan Hospital.
Transfusion Medicine
Clinical & Quality Updates
Transfusion Medicine is committed to partnering with our community to collect much needed blood products and our clinical colleagues to provide high quality transfusion therapy. A few examples of ongoing projects are focusing on the following areas:
- New Inclusive Screening Process expands Blood Donor Eligibility at the UCLA Blood & Platelet Center – Implemented the updated FDA blood donation eligibility guidelines which allow for a more inclusive blood donation process that assesses all potential donors equally while maintaining a safe blood supply. This change eliminates the FDA’s previous policy that deferred men who have sex with men from giving blood. For more information visit the Individual Donor Assessment page.
- UCLA’s New Sickle Cell Disease Center of Excellence – Implemented workflows and CareConnect tools to ensure that patients with a diagnosis of Sickle Cell Disease receive RBC genotyping prior to transfusion and phenotype-matched RBC units for transfusion. For more information visit Sickle Cell Disease Care.
- COVID-19 Convalescent Plasma – Ensuring a sufficient inventory of CCP (COVID-19 Convalescent Plasma) for the treatment of COVID-19 in patients with immunosuppressive disease or receiving immunosuppressive treatment in either the outpatient or inpatient setting. UCLA continues to provide CCP to 2-4 patients each week. If you have recently recovered from COVID-19, please consider donating CCP at the Blood & Platelet Center. For more information on donation visit Donate Blood at the UCLA Blood & Platelet Center.
- Immune-Mediated Platelet Refractoriness – Implemented platelet crossmatch testing for our patients who have immune mediated platelet refractoriness. This in-house testing ability allows our blood bank to identify these patients more expeditiously and provide compatible platelet products within a shorter TAT when compared to HLA antibody testing and provision of HLA matched platelet products from a community blood center.
- Massive Transfusion Protocol (MTP) – Leading a quality initiative to update MTP nomenclature, and to develop consistent and reliable electronic methods for documenting blood product administration during a multi-unit transfusion event (i.e. Piloting a new Epic application for documentation of massive transfusion in the trauma setting in the fall of 2024).
Research Update
The Division of Transfusion Medicine is participating in the CHIPS (CHIlled Platelet Study) study, a phase III, multicenter, randomized, double-blinded, adaptive, non-inferiority, storage duration ranging international trial in adult and pediatric patients undergoing cardiac surgery that will compare the transfusion of cold-stored platelets (stored 1-6°C) at multiple storage durations to standard room temperature platelets (stored 20-24°C). The goal of the trial is to determine whether cold-stored platelets are non-inferior (or superior) in terms of hemostatic efficacy relative to standard room temperature platelets, and, if so, to determine the maximum duration of cold storage that maintains non-inferiority to room temperature platelets. The hypothesis is that cold-stored platelets have increased hemostatic efficacy, improved safety, and are logistically superior due to their extended shelf life. Currently, this study is approaching its 2nd interim analysis, with 361 patients transfused as well as another 740 participants enrolled. UCLA is one of the top 5 centers participating in the trial with respect to number of patients engaged, with 38 patients transfused and an additional 53 enrolled.
Highlighted Publications

Congratulations to Dr. Alyssa Ziman and collaborators on their publication "Antibody Correlates of Protection for COVID-19 Convalescent Plasma Associated with Reduced Outpatient Hospitalizations."

Congratulations to Dr. Dawn Ward and collaborators on their publication "Women and Non-White People among Lasker Award Recipients from 1946 to 2022: Cross Sectional Study."
Immunogenetics
UIC Collaboration with UCLA Renal and Hematopoietic Stem Cell Transplant (HPSC) Teams Leads to First Successful "Retroactive" Tolerance Induction in a Kidney Transplant Recipient
The UCLA Immunogenetics Center (UIC) is excited to collaborate with Dr. Veale and the UCLA renal and hematopoietic stem cell transplant (HPSC) teams to successfully induce durable mixed chimerism and immune tolerance with delayed stem cell infusion after kidney transplant. The persistence of mixed donor-recipient immune cells (chimerism) after combined kidney and HSPC transplantation allows the withdrawal of all immunosuppressive therapy. Patients are able to enjoy uninterrupted normal functioning of the kidney graft while living free from the numerous life-long drugs typically required, helping them avoid adverse effects such as infections and cancer. UCLA and others have demonstrated a high success rate of withdrawal of immunosuppression amongst HLA-identical patients receiving simultaneous kidney and HPSC transplant.
This latest achievement is the first “retroactive” tolerance case in the world, and a tremendous breakthrough in medicine. UCLA has now performed the first ever HPSC infusion in a patient with a pre-existing kidney transplant, and at just 3 months after tolerance induction mixed chimerism was demonstrated! This new clinical treatment will greatly expand the pool of transplant candidates, removing prohibitive costs as a barrier toward participation and increasing access to underserved and diverse populations in an inclusive and equitable fashion. Retroactive tolerance is more practical than simultaneous tolerance because the recipient has already recovered from kidney transplant surgery. Timing for participation in retroactive tolerance can now be based on donor-recipient convenience, uncoupling the transplant and HPSC infusion. Logistically, retroactive tolerance lessens the burden on the patient and the hospital system, as treatments and follow up are all scheduled on an outpatient basis. Retroactive tolerance is also an important stepping stone for other solid organ transplants beyond the kidney, as heart, lung, and liver patients often recover in intensive care units and are not physically able to be transferred for image-guided radiation as part of their tolerance conditioning regimen until they are fully recovered and ambulatory.
UIC is fortunate to be part of this journey, serving as the testing laboratory to evaluate the percentage of mixed chimerism for patients receiving tolerance therapy. The laboratory’s role is to test the patient’s chimerism over time to determine if tolerance has been achieved. The Immunogenetics Team, including client services, clinical laboratory scientists, supervisors and laboratory directors, plays an essential function in performing, reviewing, interpreting, and reporting the test results.
Histocompatibility Scientist Training Program
UIC is also pleased to announce that they have reinstituted their Histocompatibility Scientist training program. This is a 12 month program that offers individuals the chance to train in the HLA laboratory to gain the hands-on skills and knowledge required to obtain the California Histocompatibility License. The first candidate was selected and initiated her training in June 2023! If any individuals would like information regarding this program or how to apply when the next recruitment phase begins, they may email the lab manager, Krystal Kendall, at [email protected]
September 2023 - Lab Stewardship
Laboratory test results inform or influence 50-70% of clinical decision-making and treatment planning (Arshoff L et al., Healthc Manage Forum. 2021). Unfortunately, studies have documented significant rates of unnecessary or inappropriate lab testing and delays in (or lack of) retrieval of lab results. Collectively, this leads to misleading test results, erroneous diagnoses, unnecessary costs to patients/health care systems and hospital-acquired anemia; this also can result in unnecessary subsequent testing (lab or other), unnecessary referrals and significant patient anxiety.
Laboratory Stewardship programs work to ensure patients receive the right test at the right time. As we as a nation pivot towards the concept of “value-based” care, effective Laboratory Stewardship programs, along with expert pathologists/laboratorians, will be critical in helping us achieve high value care.
Departmental Laboratory Stewardship programs are more successful in organizations that embrace this culture. We are fortunate and delighted that our Laboratory Stewardship efforts align with UCLA Health’s clinical institutional goals of enhancing patient safety and quality, improving access to care and ensuring financial sustainability. The Department of Pathology and Laboratory Medicine is committed to collaborative teamwork in laboratory stewardship by partnering with our clinicians, administrators and leadership.
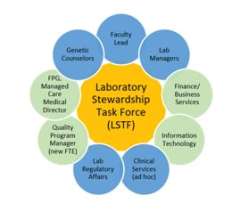
An integral part of a Laboratory Stewardship Program is leadership commitment and governance. Department leadership is pleased to be making important investments into these programs, including recently recruiting Dr. Allison Chambliss as our Director of Laboratory Stewardship and Monique Trinh as our Program Manager. UCLA Health leadership also has been extremely supportive of our programs, and has supported our governance structure, described below.
The Laboratory Stewardship Task Force (LSTF) is our lab-based group that develops and executes goals that ensure patients get the right test at the right time. LSTF composition is shown at right and this group meets twice monthly. The overarching goals of our laboratory stewardship program include:
- Improving the ordering, retrieval, and interpretation of appropriate laboratory tests, and;
- Developing, maintaining, and improving systems to provide proper financial coverage for medically necessary testing.
LSTF Co-Chairs: Dr. Allison Chambliss, Dr. Joshua Deignan, Alex Martin, PA (ASCP), MHA; Program Manager Monique Trinh, MHA

The LSTF reports quarterly to the Laboratory Stewardship Oversight Committee (LSOC). The LSOC (membership shown at left) consists of key UCLA Health leadership to provide oversight to the LSTF, assist with system-wide implementation efforts and raise requests for LSTF action.
Pathology Co-Chairs: Dr. Allison Chambliss, Dr. Omai Garner
Recent Accomplishments
Earlier this year, the LSTF announced the successful approval (by the LSOC, Ambulatory Policy Committee, and Inpatient Policy Committee) of significant revisions to Health System Policy 1309 (entitled “Laboratory Specimens - Referral to Outside Laboratories”). The main goals of these policy revisions were to 1) streamline the referral lab review/approval process to ensure compliance with the California Department of Public Health requirements, 2) reduce the inappropriate or wasteful use of referral labs by UCLA providers, and 3) enhance the utilization of available in-house testing. An additional new policy (currently under review) will establish appropriate uses of reduced rate testing offered by reference laboratories.
The revised HS 1309 policy can be found here. This policy now further solidifies the role of Pathology and Laboratory Medicine in the oversight and selection of appropriate send-out tests (and laboratories) and, in the future, will likely aid in significantly reducing the use of “miscellaneous” free-texted referral lab test orders. Reducing the “miscellaneous” orders, in turn, will allow us to better monitor our use of outside tests/labs in a continual effort to ensure the best tests and test usage for our patients.
Additionally, the LSTF has updated and digitized (via Smartsheet) the reference lab test request form (16069) for providers to request new send-out tests. Recent enhancements to the form include the addition of questions related to the number of patients who may need the test annually and the degree of physician group consensus for the medical necessity of the test. Each new submission is reviewed by the LSTF for approval prior to initiating new test builds in CareConnect. As needed, subject matter experts outside of the LSTF (including other Pathology faculty) are consulted for their expertise in various testing areas.
Ongoing Projects:
Reference Lab Optimization
Problem Statement:
High use of "miscellaneous" reference lab test orders (for tests not available in CareConnect) is inefficient and causes delays in test ordering and result retrieval.
Proposed Solution:
Identify and prioritize high-volume, high-impact, and analytically and clinically appropriate reference lab tests to be built as directly orderable and interfaced in CareConnect.
Goals:
- Develop a report to monitor miscellaneous test orders;
- Build ~150 new tests in CareConnect;
- Reduce use of miscellaneous order workflows and measure efficiency improvements.
Project Champions:
Dr. Allison Chambliss, Dr. Joshua Deignan, Dr. Alyssa Ziman, Marivic Visico, William Werre, Alex Martin, Monique Trinh
Ongoing Projects:
Inpatient Genetic Testing
Problem Statement:
Genetic testing in the inpatient setting can be problematic due to long turnaround times (e.g., results reported after discharge) and high costs that are not typically reimbursed under diagnosis-related group payment models. Results from a national survey indicate that 75% of institutions require some level of approval for inpatient genetic tests.
Proposed Solution:
Implement a CareConnect alert requiring Laboratory Medical Director consultation and approval for selected inpatient genetic test requests.
Goals:
- Ensure medical necessity;
- Ensure appropriate communication of results to patients;
- Improve access to genetic counseling and test reimbursement.
Project Champions:
Dr. Joshua Deignan, Lana Rapoport
Future Plans
- Implement CareConnect order frequency alerts for laboratory tests ordered too close in time for the same patient
- Proactively address the utilization of tests with the potential to be audited by CMS
- Contribute to national laboratory stewardship benchmarking and harmonization efforts
We are open to YOUR ideas for new laboratory stewardship projects and focus areas! Contact an LSTF co-chair or Monique, or submit to the new LSTF suggestion box!
Check out our new Laboratory Stewardship Task Force display board outside of CHS 13-262!
August 2023 - Reducing Healthcare Inequities
The Department of Pathology and Laboratory Medicine is committed to serving all patient populations and producing equitable outcomes for the patients we serve. In support of this, one tactic for the patient care component of our Office of Inclusive Excellence Plan focuses on utilizing our Blood & Platelet Center (BPC) to reduce healthcare inequities in the patient population.
Patients must receive blood transfusions from donors with compatible blood types. However, some patients may develop antibodies to antigens on transfused blood cells, making it difficult to find compatible blood units. Additionally, specific blood types are unique to particular racial and ethnic groups. Therefore, having a diverse blood supply enhances our ability to offer antigen-matched units to our diverse patients.
To achieve this, the BPC actively collaborates with community partners to increase the number and diversity of blood donors to meet the blood needs of all patients. Please read on to see how the BPC continues to expand their reach with a selection of diversity focused blood drives.
We thank the BPC for their continued efforts and all of our donors for their partnership in supporting efforts to reduce healthcare inequities in our patient population! If you know of any community partners that we should collaborate with, please send their information to the Pathology Inclusive Excellence Co-Chairs!
Hispanic Heritage Month Blood Drive - October 17, 2022-October 21, 2022 - Dr. Zhen Mei presented at the Latinx Health and Wellness Event.

Black History Month Blood Drive - February 4, 2023
Hillel’s Mitzvah Week Blood Drive - On February 24, 2023, Dr. Ziman presented at Shabbat, in preparation for Hillel’s Mitzvah Week Blood Drive.
University Muslim Medical Association (UMMA) Blood Drive - May 15, 2023-May 19, 2023 - Dr. Ward presented to the UMMA on May 16, 2023.
Asian American Pacific Islander (APPI) Month
Philipinx Blood Drive - May 19, 2023 - Sponsored by the Pilipinx Living Learning Community
Vietnamese Community Health Blood Drive - May 22, 2023-June 1, 2023
New FDA Policy
In light of the FDA’s new Individual Donor Assessment for blood donation, the BPC is preparing to implement the policy with a go-live date of September 25, 2023. The BPC plans to engage with a broader scope of demographics to diversify blood donor outreach, including that of the eligible LBGTQ Community.
Tom King, our BPC Recruiter, was recently featured in LBGTQ Nation, regarding his journey for blood donation. Read more here.
Click here to learn more about blood donation
UIC Update - Going Paperless
The UCLA Immunogenetics Center has successfully moved from paper reporting to a fully electronic (paperless) process for the majority of our HLA typing and HLA and non-HLA antibody tests, which are routinely ordered for solid organ and stem cell transplantation, including both pre and post-transplant immune assessment. Among these tests is the Single HLA Antigen Antibody test, which is UIC’s highest volume test ordered in the laboratory.
The implementation of these processes has streamlined workflows and significantly reduced the amount of time required to analyze and report test data. Not only is the reporting must faster, but removing paper from the lab has allowed us to move forward with our goals to implement a 5S system in the laboratory to enhance our organization of the workspaces. Being paperless also decreases the potential to misplace reports during the transfer from laboratory technologists to laboratory director and back to the technologist, thus improving quality systems.
UIC plans to continue these efforts by next implementing a paperless workflow for their flow cytometry crossmatches at which point, they will be 100% paperless in all reporting throughout the UCLA Immunogenetics Center.
July 2023 - Welcome to Our New Trainees & Faculty
As we begin the new academic year, I would like to provide a warm welcome to our new Residents, Fellows, and Faculty. Additionally, I would like to highlight the Farewell Ceremony awards and recipients.
New Trainees
Please join me in welcoming our new PGY1 Residents and new Post Junior Fellows, ACGME Fellows, Non-ACGME Fellows, and Clinical Post Docs! Full list here.

New Faculty

Dr. Sun Mi Choi will be joining the division of Immunogenetics as an Assistant Professor, starting August 1, 2023.
Dr. Choi graduated from LSUHSC in New Orleans with MD PhD degrees, where she conducted research in the fields of genetics and immunology. After completing Pediatric Residency at Washington University’s St. Louis Children’s hospital and Allergy/Immunology Fellowship at UCSD, she started at UCLA Immunogenetics Center to further her training in Histocompatibility and Immunogenetics. She will join the team of directors at UIC as an assistant professor to provide comprehensive and advanced testing for solid organ and stem cell transplantation. We are delighted to welcome Dr. Choi in her new role in Immunogenetics.

Dr. Xiaotang (Alison) Du will be joining the division of Anatomic Pathology as an Assistant Professor, starting July 1, 2023.
Dr. Du obtained her medical training from the Sun Yat-sen University of Medical Sciences in Guangzhou, China. She completed her AP/CP pathology residency training at the University of Texas Medical Branch, Galveston, TX.
Additionally, she completed a Surgical Pathology Fellowship at Washington University in St. Louis, MO. She has been a Gastrointestinal and Hepatic Pathology Fellow at the University of Chicago, IL since 2022. We are delighted to welcome Dr. Du in her new role in the section of GI and Liver pathology.

Dr. Alden Huang will be joining the division of Anatomic Pathology as an Assistant Professor, starting August 21, 2023.
Dr. Huang obtained his Ph.D. in Bioinformatics from UCLA. He has been a Laboratory Genetics and Genomics Fellow at UCLA since 2021. We are delighted to welcome Dr. Huang in his new role in the section of Cytogenetics.
Dr. Matthew Koo will be joining the division of Anatomic Pathology as a Staff Physician, starting July 1, 2023.
Dr. Koo obtained his medical training from the John A. Burns School of Medicine in Honolulu, Hawaii. He completed his AP/CP pathology residency training at UCLA.
Additionally, he completed a Hematopathology fellowship as well as a Surgical Pathology fellowship specializing in Soft Tissue and Bone Pathology at Stanford University He has been a practicing pathologist at Huntington Hospital since 2021. We are delighted to welcome Dr. Koo in his new role in the section of Hematopathology.

Dr. Israa Laklouk will be joining the division of Anatomic Pathology as an Assistant Professor, starting July 1, 2023.
Dr. Laklouk obtained her medical training from the University Of Tripoli School Of Medicine in Tripoli, Libya. She completed her AP/CP pathology residency training at Boston University Medical Center in Boston, MA.
Additionally, she completed a Head and Neck Pathology fellowship at Massachusetts General Hospital and a Surgical Pathology fellowship at the University of California, San Francisco, CA. She has been a faculty at the University of Wisconsin-Madison since 2021. We are delighted to welcome Dr. Laklouk in her new role in the section of Head and Neck and Genitourinary Pathology.

Dr. Manando Nakasaki will be joining the division of Anatomic Pathology as an Assistant Professor, starting July 1, 2023.
Dr. Nakasaki obtained his medical training from the Osaka University Medical School, Suita, Japan. He completed his AP/CP pathology residency training at the University of California, Irvine.
Additionally, he completed a Surgical/Bone and Soft Tissue Pathology fellowship at UCLA. He has been a Molecular Genetic Pathology Fellow at Cedars-Sinai Medical Center Los Angeles, CA since 2022. We are delighted to welcome Dr. Nakasaki in his new role in the section of Molecular Pathology.

Dr. Nicholas Stanzione will be joining the division of Anatomic Pathology as an Assistant Professor, starting July 1, 2023.
Dr. Stanzione obtained his medical training from the Geisinger Commonwealth School of Medicine in Scranton, PA. He completed his AP/CP pathology residency training at UCLA.
Additionally, he completed a Cytopathology fellowship at Stanford University Health Care and VA Palo Alto Health Care System. After training he joined Calpath Medical Associates, a private practice in the San Jose area, and most recently he has been a faculty at the VA Greater Los Angeles Health Care System since 2022. We are delighted to welcome Dr. Stanzione in his new role as a general pathologist covering Westwood as well as our affiliated Santa Monica and Northridge Hospitals.
Retirement Announcement
After 48 years of service to the UCLA Department of Pathology and Laboratory Medicine in the David Geffen School of Medicine and the UCLA Department of Biostatistics in the Fielding School of Public Health, Dr. David Gjertson, Adjunct Professor, will be retiring on June 29, 2023. Dr. Gjertson, a biostatistician, made impactful contributions in the fields of cancer, genetics, immunogenetics, laboratory studies, and transplantation medicine with over 200 peer-reviewed publications.
In May of 1975, he started working at the UCLA Tissue Typing Laboratory under the direction of Dr. Paul Terasaki who introduced him to human leukocyte antigen (HLA) testing and its important role in determining transplant compatibility between patients with end-stage renal disease and their potential organ donors. He continued to identify key transplant factors that predict chronic organ failure at the UCLA Immunogenetics Center (UIC) under the direction of Dr. Elaine Reed. He also contributed seminal findings in calculating probabilities of DNA pedigrees by incorporating measurement errors and robust estimation of HLA genotype frequencies. He subsequently contributed to the development of internationally recognized standards for the statistical interpretation of genetic evidence used in relationship testing and edited two volumes of worldwide HLA frequencies. Dr. Gjertson co-directed UIC’s reference area and is principal investigator on a long-standing contract to provide histocompatibility survey material to the College of American Pathologists. Dr. Gjertson has served numerous times as a Department of Defense and FDA panelist as well as a member of the UCLA Clinical and Translational Science Institute (CTSI) Scientific Review Committee. Since 1988, he taught introductory biostatistical courses to public health graduate students and developed consulting courses designed to enrich biostatistics doctorial students with "true-life" experiences. One of his passions was teaching SCUBA diving through UCLA Recreation and volunteering on the Los Angeles County Sheriff's Department dive team for 40 years.
Dr. Gjertson’s near 5 decades of service as an exceptional educator, scholar and collaborator has positively impacted the UCLA Immunogenetics Center and our Department and we thank him for all of the contributions he has made. Please join us in wishing him all the best for his retirement.

Farewell Ceremony
Please join me in honoring our Farewell Ceremony award recipients and graduating Trainees!
As we start anew, I look forward to our department focusing our continued excellence and advancement in clinical care, education, and research.



June 2023 - Research Updates & Graduates
The Department of Pathology & Laboratory Medicine is an integral part of the vibrant UCLA research enterprise and includes faculty members with a broad array of basic, translational, and clinical research interests.
Research & Clinical Excellence Day
Our second annual Research and Clinical Excellence Day was held on Friday, May 26, 2023. The event highlighted the ongoing basic, translational and clinical research in our department including quality, lab stewardship and education research. The event included presentations and over 20 poster displays. Thank you to our speakers, Drs. Charisse Treece, Yazhen Zhu, Dinesh Rao, Fausto Rodriguez, Ting Zhang, Nicole Valenzuela, and our keynote speaker, Dr. Gay Crooks.
The poster presentations were equally diverse. Thank you all who participated - over double from last year!
I’d also like to thank the Research Day Committee, Drs. Jonathan Said (Chair), Tom Lee, Neda Moatamed, Bogdan Pasaniuc, and Jasmine Zhou for their vision in creating a platform to showcase department research.
Save the Date! Our third annual Research and Clinical Excellence Day will be held on Friday, May 31, 2024. As you and your team present research in the upcoming year, please remember that we would love to have you present your work at our event.
Research Awards
I am proud to profile our new research awards as of July 1st. We congratulate the faculty principal investigators on their grant awards and look forward to their innovative findings and results.
The publications, grants and collaborations by our faculty underscore that UCLA is a highly interactive and collaborative environment in which interdisciplinary research flourishes. Our faculty and students continue to conduct important and meaningful basic, translational and clinical research and I look forward to learning about their future discoveries. Departmental research has continued to thrive and grow over the past decade. Our faculty publish their findings in many of the top biomedical journals such as Cell, Nature, Immunity, Molecular Cell, Nature Immunology, and the Proceedings of the National Academy of Sciences.
To date for FY23, faculty researchers generated over $4.8 million in active extramural funding, including over $1.7 million from NIH. This is double the amount of new funding compared to the same time period for last year – congratulations to all and thank you for all the grants submitted!
Graduating Staff & Clinical Trainees
As the academic year comes to a close, I would like to spotlight our graduating trainees, including staff and clinical MD/PhD trainees. We thank them for their dedication and wish them the best in their future endeavors.
Staff & Other Trainees

Cytogenetics Training Program - Jennifer Tolosa

Clinical Genetic Molecular Biologist Scientist (CGMBS) Program - Linh Lam

School of Cytology - Sharleen Decano, Vanessa Moy, Guangzhu Zhang, Jun Zhao


PA Students - Elaine Hunter & Alexander Knoester
Clinical Trainees - Please view a list of our Clinical Trainees and their future endeavors.
Post Docs - Please view a list of our Post Docs and their future endeavors.
May 2023 - Microbiology Updates
The Clinical Microbiology Lab has recently developed and launched 3 new clinical tests. These tests target recent outbreaks of emerging infections and organisms that are very difficult to perform susceptibility testing.
- In response to the recent mpox outbreak, the UCLA Clinical Microbiology lab developed and launched an mpox PCR for clinical diagnosis. We were one of the first UC labs to have the test available in-house. This test helped support the quick response to this new emerging disease.
- Klebsiella pneumoniae (a gram-negative enteric bacteria) can cause devastating systemic infection if they exhibit a "hypervirulent" phenotype. Early recognition of hypervirulence can allow for a change in clinical care and better outcomes for patients. There are very few reliable tests in the clinical lab that can recognize hypervirulence in Klebsiella pneumoniae isolates. We have developed and launched a whole genome sequencing assay that can reliably identify the hypervirulence phenotype by looking for the presence of bacterial genes associated with hypervirulence. The test has already identified numerous hypervirulent klebsiella strains from patients and had a positive impact on their care.
- Susceptibility testing for Mycobacterium tuberculosis (MTB) is important because multi-drug resistant strains are becoming more prevalent in the United States. Phenotypic susceptibility testing can take 3-5 weeks after organism identification, causing delays in appropriate therapy. We have developed and launched an MTB whole genome sequencing anti-microbial resistance test that is able to provide results in 1 week after organism identification. This test represents a new gold-standard in TB testing.
The Clinical Microbiology Lab has recently developed and launched 3 new clinical tests. These tests target recent outbreaks of emerging infections and organisms that are very difficult to perform susceptibility testing.
- In response to the recent mpox outbreak, the UCLA Clinical Microbiology lab developed and launched an mpox PCR for clinical diagnosis. We were one of the first UC labs to have the test available in-house. This test helped support the quick response to this new emerging disease.
- Klebsiella pneumoniae (a gram-negative enteric bacteria) can cause devastating systemic infection if they exhibit a "hypervirulent" phenotype. Early recognition of hypervirulence can allow for a change in clinical care and better outcomes for patients. There are very few reliable tests in the clinical lab that can recognize hypervirulence in Klebsiella pneumoniae isolates. We have developed and launched a whole genome sequencing assay that can reliably identify the hypervirulence phenotype by looking for the presence of bacterial genes associated with hypervirulence. The test has already identified numerous hypervirulent klebsiella strains from patients and had a positive impact on their care.
- Susceptibility testing for Mycobacterium tuberculosis (MTB) is important because multi-drug resistant strains are becoming more prevalent in the United States. Phenotypic susceptibility testing can take 3-5 weeks after organism identification, causing delays in appropriate therapy. We have developed and launched an MTB whole genome sequencing anti-microbial resistance test that is able to provide results in 1 week after organism identification. This test represents a new gold-standard in TB testing.
April 2023 - Inclusive Excellence Related Research
In addition to our Inclusive Excellence Committee, our department also supports inclusive excellence within our research activities. I would like to spotlight our ongoing efforts to forward inclusive excellence in the research arenas.
Anatomic Pathology
Dr. Neda Moatamed, Dr. Sarah Zhang, Dr. Erika Rodriguez, et al
Title: Clinical history of female-to-male transgender patients is needed to avoid misinterpretation of cervical Papanicolaou tests.
Cervical cancer screening is as important in female-to-male transgender (FTMT) individuals as it is in cisgender female patients. This study has examined the impact of clinical information regarding gender identity and testosterone therapy on the cytological interpretations. The findings indicate that clinical information regarding whether a subject is a FTM and/or is receiving testosterone therapy is crucial to avoiding Pap test overcalls.
This retrospective study was performed By Neda A. Moatamed, Adriana Del Barco, Stephanie Yang ,Yong Ying, Sarah Zhang, and Erika Rodriguez . After publishing the article, Dr. Sarah Zhang and Mary Levin have continued and worked closely with Beaker team on the quality improvement of the project. They introduced a system of automated flagging of the cervical Pap test of the cases who are identified as male at the time of accessioning by notification at the time of signing out. This feature can reduce the number of atypical Pap tests.
Dr. Erika Rodriguez, Dr. Precious Fortes, Dr. Neda Moatamed, Dr. Jitin Makker
Assessing Racial/Ethnic Disparities in Cervical Cancer: Prevalence of Co-Infection by Multiple HPV Genotypes and Cytologic Diagnoses (data collection phase).
Objectives: We aim to retrieve and analyze HPV results focusing on HPV co-infections and cytology and biopsy reports to evaluate racial and sociodemographic differences in HPV vaccination status and compare the risk of cervical lesions on those patients.
Specific Aims:
- Compare HPV testing, HPV co-infections and cytology results between different racial/ethnic groups and evaluate for disparities that may exist
- Determine the incidence of HPV co-infection in our UCLA population
- Evaluate the association between HPV co-infection and follow up for screening
- Determine if patients with HPV co-infection are at a higher risk of cervical lesions
Laboratory Medicine
Dr. Omai Garner
- Multi-institutional project to develop Point of Care Cardiac and Diabetes tests for underserved populations in the US. This project is developing new point of care platforms based on cell phones and implantable monitoring devices.
- A Global Health project with colleagues in the Gambia and Kenya to extend clinical microbiology testing capability. The project also includes colleagues at UCLA from infection prevention to bring best practices in clinical testing and infection prevention to low/middle income countries.
UIC
Dr. Elaine F. Reed
- They are collaborating with CEAL leaders Keith Norris and Arlene Brown entitled “Share, Trust, Organize, Partner: The COVID-19 California Alliance (STOP COVID-19 CA) Phase 3"
- Subtitle: LA-SPARTA Synergy Operation COVID-19 (LASSO COVID-19)
- Purpose: To advance mutual goals and synergistic opportunities for CEAL / STOP COVID-19 and LA-SPARTA with a focus on at-risk and underserved south LA populations disproportionately impacted by this pandemic. A translational purpose of this initiative is to enhance personal and public health education to promote improved outcomes in underserved persons at highest risks of exposure and greatest vaccine hesitancy.
- In collaboration with Michael Yeaman at the Lundquist Institute, Harbor-UCLA they identified differences in male vs female outcomes in our experimental model of MRSA bacteremia. Our submitted U19 grant application focuses in part on defining host sex contexts that drive immune protection against exploitation by MRSA.
- Their funded research in ischemia reperfusion injury, immune signatures of CMV and COVID-19 infection explore the impact of age, race, sex as predictors of outcome.
- Elaine F. Reed, in collaboration with the Women in Transplantation, has published a manuscript with Frontiers Immunology.
- Mannon RB, Reed EF, Melk A, Vinson A, Wong G, Ahn C, Davidson B, Foster B, West LJ, Tait K, Chong AS. A multi-faceted approach to sex and gender equity in solid organ transplantation: The Women in Transplantation Initiative of The Transplantation Society. Front Immunol. PMID: 36263043; PMCID: PMC9575514.
Dr. Nicole Valenzuela
- In R&D, they have two projects that are related to health equity:
- Assess utility of extended panels for detecting novel HLA antibodies and eplet reactivity in non-European patients and detecting donor specificity to non-European donor alleles.
- Evaluate different methods of HLA eplet molecular mismatch calculation in diverse patient populations, with an emphasis on inclusion of non-European patient-donor pairs for the accuracy of imputation.
Dr. Carrie Butler
- Dr. Butler is part of the HLA Dictionary Update subcommittee of ASHI and has an abstract for an oral presentation accepted at the 2022 ASHI Annual Meeting and the publication is currently in preparation. The HLA Dictionary 2022 dataset will aid in improved registry search algorithms and risk assessment of more diverse populations. The abstract title and authors are listed below:
- HLA Dictionary 2022 - Updated Serologic Prediction Models Based on Paired DNA-Serology Assignments from NMDP Registry Typing Data. Biagini1, 2, L. Gragert1, 2, , M. Maiers3, M. Milsten3, J. Kempenich3, E. Beduhn3, S. Marsh4, J. Robinson4, M. Askar5, 6, J. Houp7, M. Coppage8, C. Butler9, 10, A.F. Locke9, R. Liwski11, M. Gautreaux12, 13
Dr. Rebecca Sosa
- Dr. Sosa is currently serving as moderator for this year’s Inclusive Excellence Book/Journal Club, with the goal of highlighting the importance of diversity in science. The Club recently met to watch and discuss the film Picture A Scientist together on February 3rd, 2023, as well as distributed licenses for virtual viewing. The in-person viewing was prefaced with a powerful presentation on the current state of women in science by keynote speaker, Dr. Elaine Reed. Following the viewing the audience was split into breakout sessions with topic-specific focus groups and a broader audience-wide discussion. Participants provided excellent feedback on how the department can better provide opportunities for growth regarding diversity in science.
Research Faculty
Dr. Bogdan Pasaniuc
New Biomedical Data Science Training Program Focuses on Precision Health Equity
UCLA’s Institute for Precision Health and the Department of Computational Medicine have launched a new training program for predoctoral and postdoctoral trainees to foster a diverse, interdisciplinary environment to learn to invent and develop computational approaches and tools to deliver on the promise of precision health for everyone.
Led by Drs. Bogdan Pasaniuc and Alex Bui, the program includes faculty that cover a broad spectrum of biomedical informatics and data science research and represent 14 primary home departments from the Schools of Medicine, Engineering & Applied Sciences and Public Health; and the College of Letters & Science. Read the full article.
UCLA Health Receives $4.8M NIH Grant to Improve Genetic Estimates of Disease Risk in Diverse Populations
UCLA Health will receive a $4.8 million grant from The National Institutes of Health to develop methods that will improve genetic risk estimates – polygenic risk scores – for specific diseases in people from diverse populations and mixed ancestries.
The NIH funding comes from grants provided by the National Human Genome Research Institute (NHGRI) and the National Cancer Institute (NCI) to establish a multi-center research consortium that will pool genomic information from existing and new datasets. The researchers will then develop and evaluate methods for calculating polygenic risk scores (PRS) with an emphasis on studying people from different ancestries. Each research center may include several collaborating institutions. Read the full article.
UCLA Health Researchers Analyze LA’s ‘Stunningly Diverse’ Genetic Ancestry to Bring Ethnic Equity to Precision Medicine
Analyzing genetic ancestry data from a large genomic repository – the UCLA ATLAS Precision Health Biobank – researchers have found a highly diverse patient population that’s consistent with the global diversity of Los Angeles – one of the most ethnically diverse cities in the world and an ideal location to pursue personalized, precision medicine for underrepresented populations. Read the full article.
Session of the Pacific Symposium of Bioinformatics
This session of the Pacific Symposium on Biocomputing aims to highlight new analyses, methods, algorithms or datasets that can be applied across the continuum from research to translation to overcome disparities in precision medicine. Learn more about the Pacific Symposium of Bioinformatics.
Other Publications:
March 2023 - Staff Recognition & Community Service
This month, I would like to spotlight our monthly Staff Recognition Program. We thank these amazing individuals for their hard work, for their dedication to our patients, our clinicians, our students/trainees, our faculty and to their team, for the outstanding quality of their work and for their consistently helpful and positive outlook.
We are fortunate to have so many dedicated staff members and such wonderful teams, all focused on our vision, which is, “We are committed to healing humankind, one patient at a time, by delivering outstanding clinical laboratory services, advancing research, educating the next generation, doing what’s right, making things better and being kind.”
Below, I’m delighted to spotlight a few of the many ways members of Pathology and Laboratory Medicine are giving back to our communities and I’m happy to recognize our employees of the month for the past year.
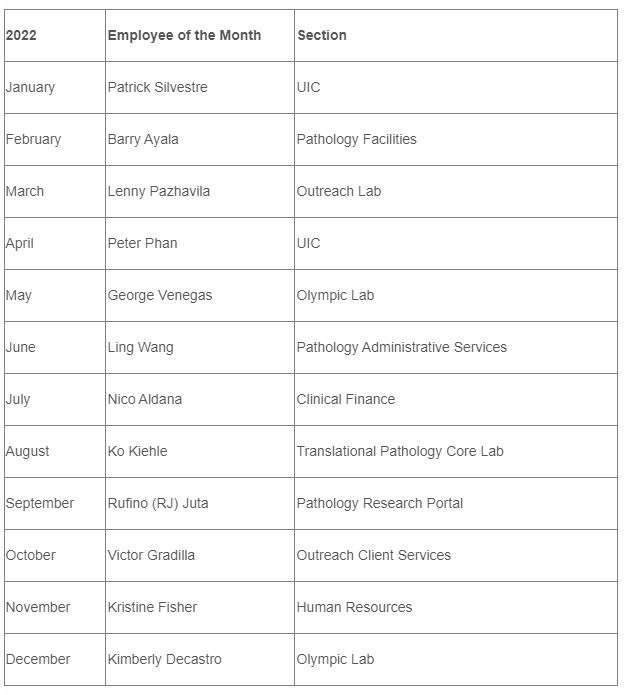
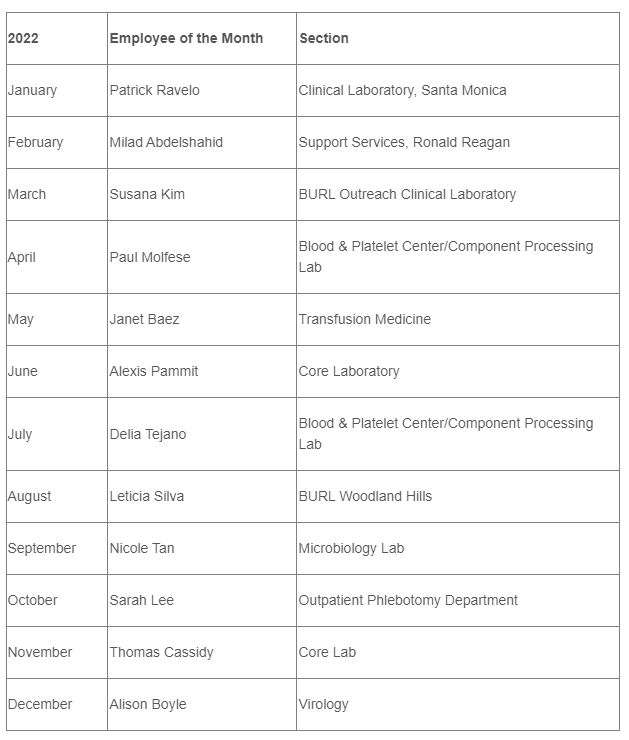
2022 DGSOM Employees of the Month
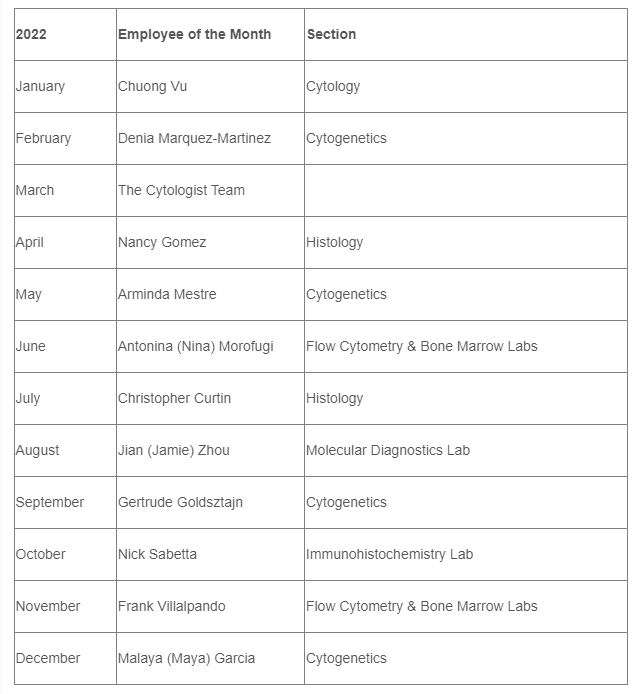
2022 AP Employees of the Month
2022 LM Employees of the Month
CORE Lab
In early 2022, Ronald Reagan Point of Care team, led by Lavita Boyd, and QA Sr. Specialists Lilian Baldwin and Khanh Andrews, sprang into action on the first day back from the holidays and set up COVID antigen testing in the 100 Medical Plaza building by January 4th. Nurses from Hospital Quality were trained to perform testing, and job aids and supplies were provided by RR Lab team. Over 600 staff were screened by the time testing wrapped up a month later.
Care Harbor Clinic
- On March 4-6, The Care Harbor Free Health Clinic hosted their annual health fair that provides free medical, dental and vision care to thousands of uninsured and underinsured people in our communities. For those who live without access to the care they need, this is a destination for help and hope. I am proud to share our department played a key role in the success of this event, as we have since 2011.
- Under the direction of Lavita Boyd, our team analyzed results for tests orders, including Wet mounts, Hemoglobin, Urine pregnancy, and Urine Dipstick. I would like to spotlight all our department volunteers: Lavita Boyd, Vladimyria C. Aquino, Candie Bautista, Ofelia Cartagena, Claire Ladan, Anna Marie Ramos, JooHee Sohn, Sonia Valadez, Emylee Villasin, and Charlene Yu.
- This event is an important way that we give back to our community and I am so grateful to our volunteers for their time and dedication to ensure the lab testing proceeded smoothly.
- Please see our event flyer for additional information and pictures of our team in action! To view the video from the UCLA Newsroom, please visit here.


Heart Walk
For the 13th consecutive year, Pathology & Laboratory Medicine fielded a team for the American Heart & Stroke Association's Heart Walk, which took place on Saturday, October 22, 2022 at the Los Angeles Memorial Coliseum. Led by Nathan Okawa, our team placed third out of 51 UCLA teams, surpassing our fundraising goal and raising $6260. UCLA Health also surpassed their goal and raised over $80,000, the most of all participating hospitals.

BLOOD & PLATELET CENTER (BPC)
- International WeLoveU Foundation Blood Drive with UCLA Blood & Platelet Center (BPC) In 2022, the BPC, WSS, WeLoveU Foundation, the Dodgers, and two blood donor centers partnered for a community blood drive at the Dodger Stadium. With a combined total of 583 donors, an unprecedented 469 Whole Blood units were collected. This number is even more impressive given the nationwide blood inventory shortage being experienced at the time. Such massive collection not only fulfilled our hospital patient transfusion needs, it enabled us to share blood products to other UC Medical Centers and neighboring hospitals.
- Resumed high school blood drives on-site while adhering to COVID guidelines.
- Resumed the Turner Internship Program during Summer 2022.
- BPC was selected as a capstone project by the Anderson School of Management, Applied Management Research Program. The AMR team will provide strategic feedback for the BPC in an effort to maximize our operations and develop a model for other hospital-based donor centers.
BRENTWOOD
- Continue C. auris testing, providing support to long term care facilities and the community.
- Surpassed over 660,000 COVID PCR tests performed since the beginning of the pandemic.
Several UCLA staff who work in Brentwood are board members of SCASM (Southern California Branch of the American Society for Microbiology), see below.

February 2023 - Hub of the Hospital
Quality Program Updates
Welcome
In July 2022, we welcomed Monique Trinh as Quality Program Project Manager. Monique is an extremely engaged team member with strong analytic, project management and people skills. She is also currently enrolled at the UCLA Fielding School of Public Health, where she is pursuing her Master of Healthcare Administration (expected to graduate June 2023). Since joining us, Monique has been instrumental in advancing QI project management throughout the department.

I am so delighted by the great strides we have taken in our quality activities over the past two years! I would like to thank and recognize our leaders who have worked tirelessly on expanding our activities and on improving the quality of our quality program:

Dr. Michelle Hickey, Director, Pathology and Laboratory Medicine Quality Improvement Program & Chair of the Quality Leadership Committee

Dr. Yuna Kang, Chair of the Quality Culture/Education/Resident Projects Committee

Dr. Alyssa Ziman, Co-Chair of the Quality Utilization and Improvement Committee

Alexander Martin, PA(ASCP), MHA, Co-Chair of the Quality Utilization and Improvement Committee
2023 Quality Improvement Day and Daljit S. and Elaine Sarkaria Keynote Lecture on Quality Improvement
Please join us for the 2023 Quality Improvement Day and Daljit S. and Elaine Sarkaria Keynote Lecture on Quality Improvement, which will be hosted virtually via Zoom from 8 a.m. to 1 p.m. on 2/10/2023. Our keynote speaker is Kate Hilton from the Institute for Healthcare Improvement. Quality Improvement Day will also feature DGSOM faculty, DGSOM trainees, and other clinicians. Detailed information about the agenda and speakers can be found on the website we created for the program. If you can join, please register here.

Pathology - Hub of the Hospital
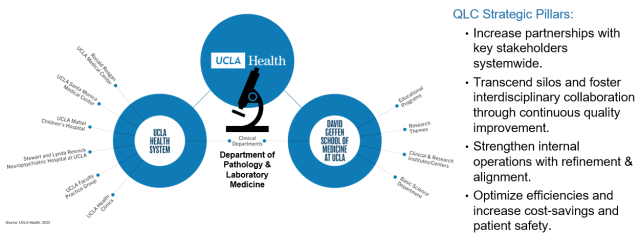
This year, we had a goal to broaden our QI footprint and partner with other Health System QI teams to improve diagnostics, operations and patient safety. Therefore, we added a new committee to our QI Program structure - the Quality Leadership Committee (QLC). The QLC was established to identify cross-departmental projects in alignment with UCLA Health, FPG, and DGSOM quality goals. In essence, Pathology and Laboratory Medicine is the hub of the hospital as samples from almost every patient seen in the hospital pass through the laboratory.
Since the specimens received are from patients from every clinical department, we think it’s important to partner with other clinical departments in quality improvement efforts. Thus, our strategic pillars at large are to:
- Increase partnerships with key stakeholders systemwide.
- Transcend silos and foster interdisciplinary collaboration through continuous quality improvement.
- Strengthen internal operations with refinement & alignment.
- Optimize efficiencies (patient safety, operations and workflow improvements, or increase cost-savings).
Current Projects
We have identified four projects to support this fiscal year:
1. Develop test order pathways for the first diagnosis of multiple sclerosis
This project is led by Dr. Michelle Hickey (Immunogenetics) and Dr. Melle Reider-Demer (Neurology). The project team consists of Dr. Michelle Hickey (Immunogenetics), Dr. Melle Reider-Demer (Neurology), and Dr. Kevin Patel (Neurology). Together, they are working to develop standard test order pathways for the first diagnosis of Multiple Sclerosis. The project endeavors to enhance patient safety and quality, improve access to care, and ensure financial sustainability. The Department is providing project management and data analysis support to achieve the following goals:
- (a) Retrospective review of test/scan utilization in first diagnosis of MS to create test ordering pathway.
- (b) Work with key stakeholders to refine test ordering pathway and create an educational process for training faculty/staff/trainees.
- (c) Evaluate impact.
2. Improving discrete reporting of clinical/diagnostic data for transplant patients
This project is led by Dr. Michelle Hickey (Immunogenetics). The project team consists of Dr. Michelle Hickey (Immunogenetics), Dr. John Zuckerman (AP), Dr. Jitin Makker (AP), and Dr. Erik Lum (Nephrology). Together, they are developing a dashboard to efficiently synthesize and analyze quality assessment information. The project endeavors to enhance patient safety and quality. The Department is providing project management and informatics support to achieve the following goals:
- (a) Improve clinical ordering of appropriate serum testing at the time of renal biopsy (to comply with current national guidelines).
- (b) Create a Tableau dashboard of data for patient management and QA needs.
3. Increase blood culture fill volumes
This project is led by Dr. Omai Garner (LM) and Allison Nomura (LM). The project team consists of Jennifer Zhang and Jonathan Aquino. Together, they are working to develop methodologies to increase blood fill volumes, increasing the accuracy of sepsis diagnosis. The project endeavors to enhance patient safety and quality, improve access to care, and ensure financial sustainability. The Department is championing the project with leadership, providing financial support for additional staffing for additional data collection, project management, and informatics support to achieve the following goals:
- (a) Improve data collection to improve data from our middleware.
- (b) Interventions – a. physically mark blood collection bottles with “fill line”; b. targeted education to providers who are consistently collecting low blood volumes.
- (c) Education – general education and onboarding education.
- (d) Consistent, constant feedback to providers regarding the blood volumes they are collecting.
4. Reference lab optimizations
This project is led by William Werre (LM), Alex Martin (AP), Marivic Visico, Dr. Josh Deignan (AP), and Dr. Alyssa Ziman (LM). The project team consists of the Lab Stewardship Task Force, Lab Support Services Management Team, and Dr. Eric Cheng (CMIO). Together, they are working to streamline the development of built tests to enhance ordering practices for reference laboratories. The project endeavors to enhance patient safety and quality and ensure financial sustainability. The Department is providing project management and informatics support to achieve the following goals:
- (a) Will create specific new test builds for ~150 tests, to create additional discrete data fields and to limit provider choices to approved non-UCLA reference labs.
- (b) Create a Tableau dashboard to be able to monitor outside lab usage.
- (c) Monitor data to determine if any testing should be brought in house, consolidated, or investigated further for appropriateness.
- (d) Monitor impact of interventions (utilization of test builds versus Misc. Ref Lab orderable, TAT, troubleshooting/result management by send out staff, etc).
Updates & Events
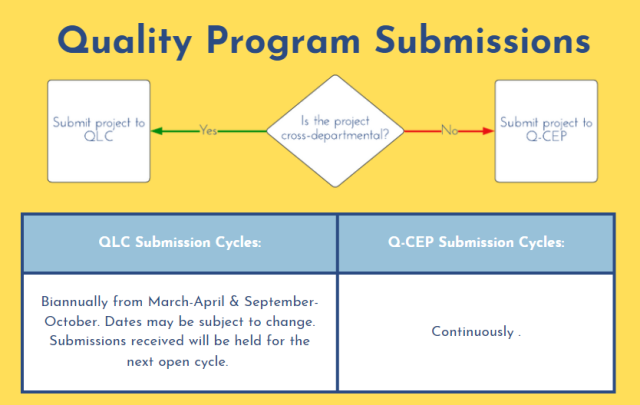
Enhancements have been made to the submission processes for QLC and Q-CEP, as outlined above. From now on, submissions can be submitted via the links below:
January 2023 - Advanced Molecular Diagnostics Service Updates
Advanced Molecular Diagnostics Service (AMDS) Updates
The Advanced Molecular Diagnostics Service has been actively working on expansion of our current clinical cancer genomic offerings to support integrated clinical cancer care for the UCLA Health System, and to identify, support and advance UCLA translational research into clinical use and technology transfer.
Team Expansion
In the last 2-3 years, the Advanced Molecular Diagnostics Service has significantly expanded the team and recruited 11 new employees:
- Liying Zhang, MD, PhD, Director, Advanced Molecular Diagnostics Service
- Cyriac Kandoth, PhD, Director, Clinical Bioinformatics, Molecular Diagnostics
- Fumin Lin, PhD, Associate Director, Clinical Cytogenetics Laboratory and Molecular Diagnostics Laboratories (MDL)
- Hua (Helen) Jin, PhD, Manager, Molecular Diagnostics Laboratories (MDL)
- Layal Abi Farraj, PhD, Clinical Variant Scientist
- Xiavan Roopnarinesingh, PhD, Software Engineer
- Mahnoosh Rahimi, MS, Clinical Variant Scientist
- Ian Atol, MS, Software Engineer
- Beau Gray, CLS
- Yuqing Zoe Zhang, PhD, CLS
- Hailey Choi, PhD, CLS
Promotions/Appointments
- Thien Huynh, Sr. Specialist, R&D
- Sze Man (Annabella) Leung, Sr. Specialist, Specimen Accession and Processing
The team launched two new solid tumor assays which utilize next-generation sequencing (NGS) technology to provide comprehensive tumor profiling for detection of substitutions (SNVs), insertion and deletion alterations (indels) and copy number variants (CNVs) and structural variants (SVs, gene fusions), as well as genomic signatures including microsatellite instability (MSI) and tumor mutational burden (TMB). These assays identity genetic alterations for cancer diagnostic, prognostic and therapeutic purposes. These assays were launched in early December 2022:
- Pan-cancer Solid Tumor NGS panel (1080 genes)
- Solid Tumor Fusion Panel (145 genes)

Starting from back (left-to-right):
Back - Jian (Jamie) Zhou, MS (CLS); Josh Deignan, PhD (Associate Director); Xiavan Roopnarinesingh, PhD (Bioinformatics Engineer); Layal Abi Farraj, PhD (Variant Scientist);
Cyriac Kandoth, PhD (Bioinformatics Director); Ian Atol, MS (Bioinformatics Engineer); Alden Huang, PhD (Fellow); Thien Huynh (Sr. CLS Specialist);
Svetlana (Lana) Rapoport (ISS Application Analyst, Beaker); Mahnoosh Rahimi, MS (Variant Scientist); Sze Man (Annabella) Leung (Sr. CLS Specialist); Fumin Lin, PhD (Associate Director);
Front - Sung-Hae (Sue) Kang, PhD (Associate Director); Leila Youssefian, PhD (Fellow); Liying (Lily) Zhang, MD, PhD (Director); Hua (Helen) Jin, PhD (Manager); Yan Li (Sr. CLS Specialist)