UCLA researchers have developed a new treatment method using a tiny nanocapsule to help boost the immune response, making it easier for the immune system to fight and kill solid tumors.
The investigators found the approach, described in the journal Science Translational Medicine, increased the number and activity of immune cells that attack the cancer, making cancer immunotherapies work better.
“Cancer immunotherapy has reshaped the landscape of cancer treatment,” said senior author of the study Jing Wen, assistant adjunct professor of microbiology, immunology, & molecular genetics at the David Geffen School of Medicine at UCLA and a scientist at the UCLA Jonsson Comprehensive Cancer Center. “However, not all patients with solid tumors respond well to immunotherapy, and the reason seems to be related to the way the cancer cells affect their surroundings.”
Cancer cells produce a lot of lactate, Wen explained, which creates an environment around the solid tumor that makes it difficult for the immune system to work effectively against the cancer.
Although there have been efforts to reduce the levels of lactate with different drug inhibitors, these methods tend to also disrupt the metabolism of healthy cells, which can cause severe side effects.
To find a way to alleviate immune dysfunction around the tumor without hurting healthy cells, Wen and the team looked to create a tool to deliver drug inhibitors directly, to degrade lactate around and within solid tumors.
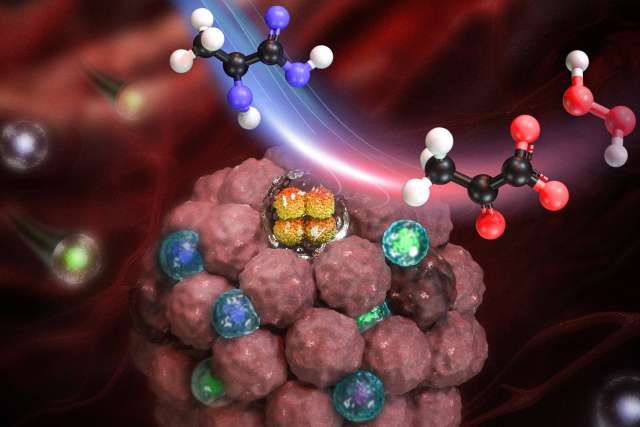
To achieve that goal, the team developed a treatment encapsulating an enzyme called lactate oxidase into a tiny nanocapsule that reduces lactate levels and releases hydrogen peroxide in the tumor.
Decreased levels of lactate are beneficial for releasing the suppression of immune response, while increased levels of hydrogen peroxide, a substance typically produced when you get injured, help recruit and activate immune cells in the tumors.
“When lactate is reduced and hydrogen peroxide is released, it makes it easier for the immune system to work against the cancer,” said Zheng Cao, first author of the study and UCLA Samueli School of Engineering doctoral candidate in the department of chemical and biomolecular engineering.
To examine the effect of nanocapsules with the lactate oxidase enzyme, the team tested the approach in mice with melanoma and triple-negative breast cancer and performed tumor growth measurement, survival curve analysis, RNA sequencing, and immune cell population analysis. The team found that reducing lactate and producing hydrogen peroxide encouraged immune cells to enter the tumor, increasing the number and activity of immune cells that attack the cancer by 2 to 5-fold.
“We found lactate oxidase nanocapsules helped prevent the immune system from being weakened and overcome the immune suppression caused by the tumor,” Cao said. “Moreover, this dual-action approach improved the success of a specific type of cancer immunotherapy treatment called immune checkpoint blockade and we believe it could be an effective strategy to help make cancer immunotherapy more effective.”
“Built on the drug delivery platform we have developed at UCLA Samueli School of Engineering, this work afforded great translational potentials towards more effective cancer immunotherapy, which may bring new hopes for cancer patients,” said Yunfeng Lu, professor emeritus of chemical and biomolecular engineering at UCLA engineering.
The researchers will further explore the impact of lactate oxidate nanocapsules on enhancing the therapeutic effectiveness of chimeric antigen receptor (CAR) T-cell therapy for solid tumors. CAR T-cell therapy is a type of cellular immunotherapy designed to modify T cells, enabling them to recognize and attack cancer.
Along with Wen and Lu, another senior author of this study is Jimin Guo, a former postdoc scholar in the department of microbiology, immunology, & molecular genetics.
This work was supported in part by grants from the UCLA ENN Center for Nanomedicine and Energy Conversion, the National Institutes of Health, and the National Center for Advancing Translational Science.