Mice fed a diet high in fat, cholesterol and calories, akin to the Western diet, had higher measures of blood lipids associated with elevated levels of inflammation, a new UCLA study finds. Researchers also identified clues to how the microbiology of the intestinal tract impacts disease-causing inflammation, suggesting that targeting the mucus interface between gut bacteria and the cells of the small intestine may be a novel means of preventing systemic inflammation.
The study was published today in the Journal of Lipid Research.
Inflammation is an important process that protects the body from invading infections and toxins. But in individuals who are successfully treated for HIV to the point that their viral load is no longer detectable, the continuing low-grade inflammation in the cells of the intestine contributes to the increased risk of heart attack or stroke in such people. These individuals have been found to have a “leaky gut” with more gut bacterial products in their blood such as the potent pro-inflammatory bacterial product known as lipopolysaccharide, or LPS, which promotes systemic inflammation that can accelerate the disease in arteries that leads to heart attack and stroke.
UCLA researchers previously used mouse models of treated HIV to study this problem. They found that adding a tomato concentrate called Tg6F to the western diet of the mice improved their “leaky gut” and significantly reduced systemic inflammation in the mice[1]. Tg6F contains a peptide mimetic of the main protein in HDL (“good cholesterol”).
To learn more about how diet is related to inflammation, researchers led by Pallavi Mukherjee fed half a group of mice a typical “western diet,” high in fat, cholesterol and calories, while the rest were fed the normal mouse diet, known as a “chow diet,” which is low in fat, cholesterol and calories. The researchers examined dietary phospholipids to identity causes of the associated systemic inflammation.
Normal phospholipids do not induce inflammation, but oxidized phospholipids are known to often induce a strong inflammatory response. Researchers suspected that the high-fat western diet might contain high levels of oxidized phospholipids accounting for the ability of this diet to induce systemic inflammation. Surprisingly, they found the western diet contained very low levels of oxidized phospholipids, while the low-fat chow diet contained much higher levels of oxidized phospholipids.
Dr. Alan M. Fogelman, chair of the department of medicine at the David Geffen School of Medicine at UCLA said, “we studied the portion of the small intestine known as the jejunum, because it is very actively involved in the uptake of dietary fats.”
The investigators focused on a thin mucous layer that is the interface between the bacteria in the lumen of the jejunum and the cells on the surface of the jejunum that take up the dietary components. The jejunum relies on antimicrobial peptides and proteins in this thin mucous layer to keep the bacteria in the lumen of the intestine from interacting with its cells.
In mice fed the western diet, the jejunum contained high levels of oxidized phospholipids compared to those in mice fed the chow diet. They also had low levels of antimicrobial peptides and proteins compared to mice fed the chow diet. Consistent with the decrease in levels of antimicrobial peptides and proteins, the number of bacteria and the levels of LPS increased in jejunum mucus from mice fed the western diet. The permeability of the jejunum also increased in mice fed the western diet.
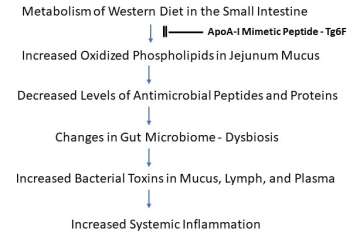
The lymph draining from the jejunum of mice fed the western diet contained increased levels of LPS as did the blood of these mice. LPS is known to induce inflammation in both mice and humans, and markers of systemic inflammation were increased in the mice fed the western diet.
Researchers hypothesized that the western diet-mediated changes were due to the formation of oxidized phospholipids in the jejunum. To test this hypothesis, they added oxidized phospholipids to segments of jejunum taken from mice on the chow diet. This reproduced the changes in gene expression seen in the jejunum of mice fed the western diet, which would account for the decrease in antimicrobial peptides and proteins on this diet.
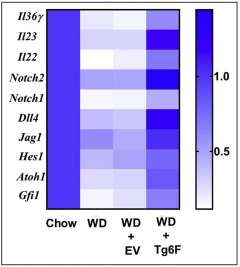
The investigators found adding Tg6F to the western diet reduced the levels of oxidized phospholipids in jejunum mucus. Additionally, it prevented the decrease in antimicrobial peptides and proteins, prevented the increase in bacteria and LPS in jejunum mucus, prevented the increase in jejunum permeability, and reduced LPS in lymph and blood, which reduced markers of systemic inflammation.
The authors say their findings suggest that targeting the mucus interface between the bacteria and the cells of the small intestine with peptide mimetics of the main protein in HDL may be a way of preventing systemic inflammation.
The study’s authors are Pallavi Mukherjee, Arnab Chattopadhyay, Victor Girjalva, Nasrin Dorreh, Venu Lagishetty,Jonathan P. Jacobs, Bethan L. Clifford, Thomas Vallim, Julia J. Mack, Mohamad Navab, Srinivasa T. Reddy, and Alan M. Fogelman of UCLA; Jonathan P. Jacobs of UCLA and Veterans Administration Greater Los Angeles Healthcare System Los Angeles.
GRANT SUPPORT
This work was supported in part by US Public Health Service Research Grant 1R01 HL148286 (A.M.F.) and the Laubisch, Castera, and M.K. Grey Funds at the University of California at Los Angeles. J.P.J. and V.L. were supported by VA CDA2 IK2CX001717 and NIH/NIDDK P30 DK041301.
CONFLICT OF INTEREST
A.M.F, M.N., and S.T.R. are principals in Bruin Pharma and A.M.F. is an officer in Bruin Pharma.
[1]AIDS 2021; 35:543-553 PMID:33306550; Metabolism 2021 Nov;124:15488.doi.10.1016/j.metabol.2021.154888. Epub 2021 Sep 9. PMID:34509494



