The Growth Plate
The growth plate is one of the few tissues in vertebrates in which cells are stratified according to their differentiation status. This makes it possible to use phenotypic analysis of mice to investigate the functions of genes in specific aspects of commitment, maintenance, proliferation, differentiation, and survival.
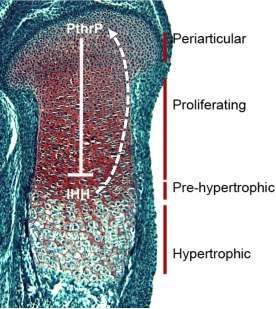
Most skeletal elements develop through endochondral ossification, in which a condensation of mesenchymal cells forms, differentiates into chondrocytes, and eventually forms a growth plate. The growth plate contains chondrocytes that are stratified according to their differentiation status. The pericarticular cells are slowly proliferating and produce less cartilage-specific matrix than do more differentiated cells. The proliferating chondrocytes are rapidly dividing cells, and their planes of cell division are oriented such that they form columns of chondrocytes. This leads to elongation of the developing skeletal element. Cells are maintained in the proliferative phase by the action of PthrP, which is expressed in the periarticular cells but diffuses through the proliferative zone. Chondrocytes most distal to the source of PthrP are no longer under its influence, and therefore become post-mitotic. Once they do this, they start to express Indian Hedgehog. They also begin to terminally differentiate, marked by the onset of hypertrophy. IHH produced by the pre-hypertrophic chondrocytes diffuses back to the periarticular cells to maintain PthrP expression. This negative feedback loop maintains the length of the growth plate.
Our laboratory is investigating how Bone Morphogenetic Proteins (BMPs) regulate growth plate formation and maintenance. We have found that BMP signaling is essential for the initial formation of chondrocyte condensations. Once a growth plate forms, we found that BMP signaling is essential for the induction of IHH. This induction appears to be direct and mediated by canonical BMP pathways.
Suggested Reading:
Karsenty G, Kronenberg HM, Settembre (2009) Genetic Control of Bone Formation. Ann Rev Cell and Devel Biol 25: 629-648.
Song B, Estrada KD, Lyons KM (2009) Smad Signaling in Skeletal Development and Regeneration. Cytokine Growth Factor Rev. 20:379-88.
Retting, KN, Song B, Lyons KM (2009) BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136:1093-1104