Research
Adipocytes are important players in the acute lymphoblastic leukemia (ALL) microenvironment
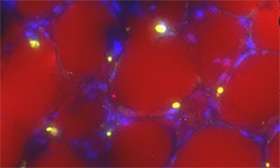
Using mouse and tissue culture models, we have shown that obesity directly impacts the progression and treatment outcome of ALL. Mice which are prone to develop ALL do so earlier when they are made obese with a high-fat diet. Further, obese mice with leukemia have a poorer response to chemotherapy. We have found that fat cells protect ALL cells from multiple chemotherapies, through a variety of mechanisms. Because fat cells are found in the bone marrow, which is generally accepted to be a protective microenvironmental niche of ALL cells, this protection could contribute to poorer outcomes in patients. Further, we discovered that adipocytes attract ALL cells through chemokine secretion, which could result in ALL cells migrating into adipose tissue where they are protected from chemotherapy and can act as a reservoir for relapsing ALL cells. We are working to understand the two-way communications between ALL cells and adipocytes, and explore strategies to block these effects.
Publications:
- J Tucci*, W Alhushki*, T Chen, X Sheng*, Y-M Kim, SD Mittelman Switch to Low-Fat Diet Improves Outcome of Acute Lymphoblastic Leukemia in Obese Mice
Cancer & Metabolism, 2018 (In Press) - JW Behan*, JP Yun, MP Proekter, EA Ehsanipour*, A Arutyunyan, AS Moses*, S Louie, A Butturini, N Heisterkamp, SD Mittelman
Adipocytes Impair Leukemia Treatment in Mice
Cancer Research 69:7867-74, 2009. PMCID: PMC2756308 - JP Yun, JW Behan*, N Heisterkamp, A Butturini, L Klemm, L Ji, J Groffen, M Müschen, SD Mittelman
Diet-Induced Obesity Accelerates Acute Lymphoblastic Leukemia Progression in Two Murine Models
Cancer Prevention Research, 3(10):1259-64, 2010. PMCID: PMC2955776 - R Pramanik, X Sheng*, B Ichihara, N Heisterkamp, SD Mittelman
Adipose TissueAttracts and Protects Acute Lymphoblastic Leukemia Cells from Chemotherapy. Leukemia Research, 37:503-9, 2013. PMCID: PMC3622767 - X Sheng*, J Tucci*, JH Parmentier, L Ji, J Behan*, N Heisterkamp, SD Mittelman
Adipocytes Cause Leukemia Cell Resistance to Daunorubicin via Oxidative Stress Response
Oncotarget, 7(45):73,147-59, 2016. PMCID: PMC5341969
Adipocytes provide fuels to acute lymphoblastic leukemia cells
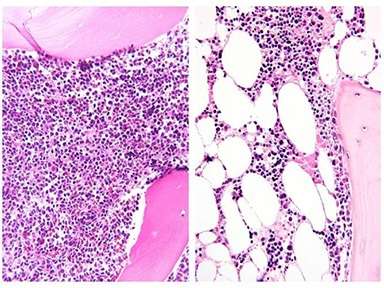
Bone marrow before and after induction chemotherapy: Bone marrow biopsy from an adolescent patient at diagnosis of ALL (left) and after one month of chemotherapy (right). Marrow is full of ALL cells at diagnosis, but these are largely replaced by large adipocytes (white ovals)
The main job of adipocytes is to store fuels and provide them to the body in times of need. While this is primarily in the form of free fatty acids (FFA), adipocytes also release amino acids. This is important because ALL cells (and other cancer cells) need amino acids for metabolismand proliferation. ALL cells in particular are dependent on extracellular asparagine and glutamine—a fact which is exploited by the use of the chemotherapy L-asparaginase, which metabolizes these two amino acids. We have shown that fat cells release asparagineand glutamine, thereby allowing nearby ALL cells to avoid the cytotoxic effects of L-asparaginase. We are working with collaborators, Claudia Scotti, to evaluate new L-asparaginase formulations which could improve efficacy, particularly in adipocyte rich microenvironments.
We have also discovered that adipocytes and ALL cells participate in a two-way communication; ALL cells induce changes in adipocyte morphology and function, and adipocytes in turn release free fatty acids (FFA), which are used as a fuel for ALL cells. In this project, we are working to further understand these effects. We are investigating how ALL cells, in the context of chemotherapy, affect adipocytes, both in culture and in mice and humans. We are also focusing on how adipocyte-derived FFA affect ALL cell growth and survival. We have developed novel techniques to perform these studies, including intravital imaging techniques to visualize bone marrow adipocyte and ALL interactions, and single cell lipidomics techniques to elucidate the effects of adipocytes on ALL cell metabolism. These studies will increase our understanding of how leukemia interacts with its microenvironment. They will also improve our understanding of the relationships between ALL and obesity, and they have the potential to change the way we treat cancer in our increasingly overweight population. These studies are supported by the NIH/NCI R01CA201444.
- EA Ehsanipour*, X Sheng*, JW Behan*, X Wang, A Butturini, VI Avramis, SD Mittelman
Adipocytes Cause Leukemia Cell Resistance to L-Asparaginase via Release of Glutamine
Cancer Research, 73:2998-3006, 2013. PMCID: PMC3684066 - JH Parmentier, M Maggi*, E Tarasco, C Scotti, VI Avramis, SD Mittelman
Glutaminase Activity Determines Cytotoxicity of L-Asparaginases on Most Leukemia Cell Lines
Leukemia Research 39(7):757-62, 2015. PMCID: PMC4458142 - J Tucci*, K Margulis, C Hsu, W Dixon*, RN Zare, SD Mittelman
Using stable isotope lipidomics to identify adipocyte-induced alterations in the acute lymphoblastic leukemia lipidome
Proceedings of the American Association for Cancer Research Annual Meeting, abstract #3294, 2016
Sequestration and deactivation of chemotherapies by adipocytes in the leukemia microenvironment
Fat cells are known to absorb some drugs, making dosing difficult in obese patients. We found that adipocytes absorb the important chemotherapy, vincristine, which alters its pharmacokinetics in preclinical models. This could potentially contribute to the poorer treatment outcomes observed in obese patients with ALL, as well as a number of other cancers in which vincristine is used.
We recently discovered that fat cells not only absorb the anthracycline, daunorubicin (DNR), but they break it down to an inactive form. This depletes local levels of the active drug, protecing nearby ALL cells from this chemotherapy. This is the first demonstration, to our knowledge, that fat cells can metabolize and deactivate a therapeutic drug. Based on this, we are working to elucidate how fat cells do this, and what can be done about it. We are looking into which enzymes in adipocytes contribute to their ability to breakdown anthracyclines like DNR, and exploring strategies to block them. We are using mouse models to determine how adipocytes in vivo alter systemic DNR availability, as well as that in the bone marrow and other ALL microenvironments. We are also exploring how clinical variables such as age and gender alter these effects. Finally, we are planning a limited sampling pharmacokinetic (PK) study in lean and obese children, to estimate DNR and DNR-ol plasma and intracellular exposure during ALL therapy. These studies will increase our understanding of how the leukemia microenvironment can contribute to treatment failure, particularly in the obese state. Findings could lead to improved strategies for anthracycline dosing and monitoring in children and adults. These results, along with our previous studies on how adipocytes affect vincristine and L-asparaginase PK, will lay the groundwork for a personalized dosing study of Induction chemotherapies in children with ALL. These studies are supported by the NIH/NCI R01CA213129.
Publications:
- JW Behan*, VI Avramis, JP Yun, S Louie, SD Mittelman
Diet-Induced Obesity Alters Vincristine Pharmacokinetics in Mice
Pharmacological Research, 61:385-390, 2010. PMCID: PMC2848885 - X Sheng*, J-H Parmentier, J Tucci*, H Pei, O Cortez-Toledo, CM Dieli-Conwright*, MJ Oberley, M Neely, E Orgel, SG Louie, SD Mittelman
Adipocytes Sequester and Metabolize Daunorubicin
Proceedings of the American Association for Cancer Research Annual Meeting, abstract #2960, 2017 - X Sheng*, J-H Parmentier, J Tucci*, H Pei, O Cortez-Toledo, CM Dieli-Conwright*, M Oberley, M Neely, E Orgel, SG Louie, SD Mittelman
Adipocytes Sequester and Metabolize the Chemotherapeutic Daunorubicin
Molecular Cancer Research, 15(12):1704-1713, 2017
Clinical research projects
In addition to our basic research projects, the Mittelman Lab is integrated with clinical research studies. This helps to ensure that our findings are relevant to patients, and allows us to directly test strategies to improve patient outcomes. A collaborative partnership was therefore established between the Mittelman Lab and the clinical research program of Dr. Etan Orgel, a leukemia and supportive care clinician-scientist at Children’s Hospital Los Angeles. This collaboration has fostered a pathway for rapidly testing of laboratory-generated findings in patients and vice-versa and has led to multiple clinical trials and key findings.
We have described the rapid and substantial decline in vertebral bone density that occurs in children during the first month of ALL treatment, as well as the surprisingly high increase in body fat (20-30%) that accumulates over the same period. We have also tested a vitamin D intervention to determine whether this could help spare vertebral bone density during the initial phases of chemotherapy in children with ALL.
In our studies, we have also observed that nearly half of ALL patients are overweight or obese at diagnosis, and that obesity at the time of diagnosis is associated with a higher likelihood of poor response to chemotherapy as evidenced by persistent leukemia (minimal residual disease) after induction therapy. Together, these data show that body fat is a significant risk factor for ALL treatment failure, and that its negative effects are evident within the first month of treatment. Recent laboratory and clinical data illustrates the ability of diet restriction and physical activity to improve chemotherapy efficacy, reduce treatment-related toxicities and better overall quality of life. Based on this, we have launched the Improving Diet and Exercise in Acute Lymphoblastic Leukemia (IDEAL Weight in ALL) trial (NCT02708108). This study tests a complete personalized dietary and exercise intervention for pre-adolescents, adolescents and young adults newly diagnosed with B-precursor ALL ("pre-B ALL") that aims to reduce fat gained during induction therapy and thereby improve treatment response, toxicity rates, and quality of life. Dr. Orgel and Mittelman designed the study together within this collaborative framework; the study is being performed at Children’s Hospital Los Angeles under the leadership of Dr. Orgel as Principal Investigator; the Mittelman Laboratory is the lead study laboratory for the clinical trial, and is performing the embedded laboratory analyses.
Publications:
- AP Vidmar*, R Pretlow, C Borzutzky CP Wee, S Fox, C Fink, SD Mittelman An Addiction Model Based Mobile Health Weight Loss Intervention in Obese Adolescents
Pediatric Obesity, 2018 (In Press) - E Orgel, J Tucci*, W Alhushki*, J Malvar, R Sposto, CH Fu, DR Freyer, H Abdel-Azim1, SD Mittelman1
Obesity is Associated with Persistence of Residual Leukemia Following Induction Therapy for Childhood B-Precursor Acute Lymphoblastic Leukemia
Blood 124(26):3932-8, 2014/ 1Co-senior authors - E Orgel, NM Mueske, TAL Wren, V Gilsanz, AM Butturini, DR Freyer, SD Mittelman
Early Injury to Cortical and Cancellous Bone from Induction Chemotherapy for Adolescents and Young Adults Treated for Acute Lymphoblastic Leukemia
Bone, 85:131-7, 2016 - E Orgel, NM Mueske, R Sposto, V Gilsanz, DR Freyer, SD Mittelman
Limitations of body mass index to assess body composition due to sarcopenic obesity during leukemia therapy
Leukemia & Lymphoma, 2016 [Epub ahead of print, PMID 26818609] - E Orgel, NM Mueske, R Sposto, V Gilsanz, TAL Wren, DR Freyer, AM Butturini, SD Mittelman
A Randomized Controlled Trial Testing an Adherence-optimized Vitamin D Regimen to Mitigate Bone Change in Adolescents Bting Treated for Acute Lymphoblastic Leukemia
Leukemia & Lymphoma, 58(10):2370-8, 2017 PMCID: PMC5489365 - MH Lin*, JR Wood, SD Mittelman, DR Freyer
Institutional Adherence to Cardiovascular Risk Factor Screening for Young Survivors of Acute Lymphoblastic Leukemia
Journal of Pediatric Hematology and Oncology 37(4):e253-7, 2015
Adipose tissue inflammation
In addition to studies on childhood leukemia, the Mittelman Lab has been interested in understanding how adipose tissue inflammation contributes to disease, including cancer. We have used preclinical models to investigate the mechanisms of immune cell infiltration and activation in adipose tissue. We also collaborate with clinical researchers to evaluate human adipose tissue for inflammation as an intervention outcome. We are currently collaborating with the laboratory of Christina Dieli-Conwright, who is performing exercise interventions in breast cancer survivors with the goal of improving adipose tissue inflammation and hopefully risk of cancer recurrence.
Publications:
- JW Behan*, EA Ehsanipour*, R Pramanik, X Sheng*, Y-M Kim, Y-T Hsieh, SD Mittelman
Activation of Adipose Tissue Macrophages in Obese Mice does not Require Lymphocytes
Obesity, 21:1380-8, 2013. PMCID: PMC3742678 - CM Dieli-Conwright*, J-H Parmentier, N Sami, K Lee, D Spicer, WJ Mack, F Sattler, SD Mittelman
Adipose Tissue Inflammation in Breast Cancer Survivors: Effects of a 16-week Combined Aerobic and Resistance Exercise Training Intervention Breast Cancer Research and Treatment, 168 (1): 147-57 - TL Alderete, FR Sattler, X Sheng, J Tucci, SD Mittelman, EG Grant, MI Goran
A Novel Biopsy Method to Increase Yield of Subcutaneous Abdominal Adipose Tissue
International Journal of Obesity 39(1):183-6, 2015 - TL Alderete, FR Sattler, JM Richey, H Allayee, SD Mittelman, X Sheng, J Tucci, LE Gyllenhammer, EG Grant, MI Goran
Salsalate Treatment Improves Glycemia without Altering Adipose Tissue in Non-Diabetic Obese Hispanics
Obesity 23(3):543-51, 2015. PMCID: PMC4340767
* indicates trainee