Translational Research Imaging Center (TRIC)
What is the Translational Research Imaging Center (TRIC)?
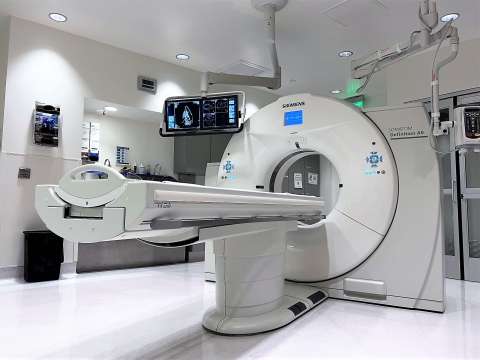
Located in the UCLA Center for Health Sciences, the TRIC is a state-of-the-art, preclinical, diagnostic and interventional imaging center. Our team of physicians, scientists, fellows, technologists and veterinary staff offer over 25 years of experience in conducting preclinical studies across the field of medicine.
The TRIC employs advanced imaging technologies to support research and education throughout the UCLA Health campus. Additionally, our experts are available to collaborate with investigators worldwide.
The UCLA TRIC resources are broadly employed to develop and test new medical devices and drug therapies and train physicians on the use of these new technologies.
Our purpose
The TRIC supports both UCLA Health and the greater scientific community in performing quality research and teaching. We aim to:
- Facilitate and participate in preclinical studies with an emphasis on ethics, safety and the humane treatment of approved and regulated laboratory animal models
- Aid the development and evaluation of novel therapies, techniques and devices
- Expand teaching and educational opportunities for staff, students and researchers across a broad cross-section of fields in medicine and science
The knowledge gained from research undertaken and supported by the TRIC drives scientific discovery toward developing lifesaving treatments for diseases and improving the quality of life for all patient populations.
Our facilities and equipment
The TRIC employs the most advanced imaging technologies currently available, such as:
- MRI: Siemens Magnetom® Prisma, a whole-body 3 Tesla (3T) MRI system with a state-of-the-art gradient system, 32-channel RF receiver and full suite of receiver coils
- CT: Siemens Somatom® Definition 64-slice dual-source scanner
- Ultrasound: Philips iU22 ultrasound system for general imaging
- X-ray angiography:
- Siemens Artis zeego angiogram suite with a robotic arm incorporating 3D-rotational and cross-sectional DynaCT capabilities along with a workstation for 3D reconstruction
- Philips Allura Xper FD10 cardiovascular X-ray system
- Vascular simulation capabilities
Facilities include:
- A Visage PACS image library
- Computer workstations capable of creating 3D visualizations from MRI, CT or X-ray images
- Interventional radiology procedure room
- Conference room that includes a space for meetings and/or didactic training
- Adjacent observation and recovery rooms
The Siemens angiography procedure room and conference room are equipped with high-definition video cameras integrated using UCLA’s video conferencing system. This allows TRIC scientists to broadcast, collaborate, teach, learn and share in real-time high-definition video and THX audio.
Our expertise
The TRIC is supported by a dedicated staff with decades of experience in collaborative research and innovation. This group of specialists includes neurointerventionalists, vascular interventionalists, abdominal and thoracic interventionalists, and MR physicists.
Proven preclinical experience
The TRIC has a long history of research leading to innovations in technology to treat a broad range of human diseases and conditions, such as:
- Brain aneurysms (Guglielmi detachable coil)
- Brain arteriovenous malformation (AVM) (Onyx® liquid embolic agent)
- Ischemic stroke (MERCI clot-retrieval device)
We have also been involved in preclinical evaluation of medical devices, including a flow-restoration device to treat acute ischemic stroke, detachable coils to treat brain aneurysm, and numerous devices used to access the human vasculature and treat cerebrovascular and peripheral vascular diseases. Examples of other devices developed or evaluated by the TRIC include:
- Ablation systems
- Cryoablation
- High-intensity focused ultrasound (HIFU)
- Irreversible electroporation (IRE)
- Microwave
- Catheter development
- Embolectomy devices
- Embolic protection devices
- Pulmonary embolism treatments
- Stents
- Vascular imaging systems
- Vascular occlusion
- Vascular sealants/vessel-closure devices
- Vena cava filters
Model development for neurological and vascular research
We have extensive experience with in vivo research models of human disease using approved and regulated laboratory animals. Our team can:
- Create surgical and interventional in vivo models
- Identify applicable animal models of human disease and anatomy
- Replicate published models from scientific journals
Furthermore, the advanced imaging capabilities available at the TRIC allow accurate and rapid model characterization and validation using minimally invasive techniques.
Utilizing human cadavers as research models
We can source serologically tested human cadaver models with disease states of interest for preclinical research studies or bioskills clinical training from the UCLA Donated Body Program. Cadavers offer a full-body vascular model for device testing, physician training and clinical procedure/application workshops.
Employing medical simulators for interventional techniques
The vascular replicator available at the TRIC represents a specialized neurovascular flow system offering an extraordinarily realistic hemodynamic environment. This system provides a vascular model capable of evaluating neurovascular devices meant for the treatment of strokes, AVMs, brain aneurysms and fistulas. The replicator yields highly reliable results.
Our services
We specialize in preclinical studies involving approved large-animal research models and human cadavers. Our staff works in conjunction with your product development team to offer customized support to meet your project needs. This includes assistance in selecting the right model for your project and support in developing experimental protocols and reports.
Our facilities and equipment enable preclinical imaging services for neurologic or vascular research models, medical device evaluation and development, and physician-training workshops. These services encompass early-stage feasibility projects through non-GLP safety studies and physician training:
- Product development studies: We support product development teams by performing early prototype evaluations, iterative testing, 2nd generation evaluations, end-user validation and non-GLP preclinical studies.
- Surgical and interventional services: Our skilled professionals can create surgical and interventional models of specialized anatomy in large, approved laboratory animals to test your product, provide valuable feedback on product performance and optimize treatment steps in preparation for human clinical cases.
- Physician training: We can host physician training on your device in approved human cadaver, animal or benchtop models prior to clinical use.
- Clinical products for studies: Our staff can source clinical products required for controls or other aspects of a protocol.
- Histopathology and bioanalytical testing: The TRIC collaborates with industry experts to coordinate the necessary support to assist with your research and training goals.
In addition to imaging services and procedure rooms for device evaluations, we can accommodate simultaneous hands-on training and didactic sessions in accompanying conference rooms. Our staff can support your efforts from protocol development to assisting with catering services. Our goal is to ensure that your research or physician-training sessions run smoothly from beginning to end.
Frequently asked questions
Below are some common inquiries concerning our capabilities and services. If you need additional details or clarification, please contact us.
How can I obtain a quote for a preclinical study or training workshop?
Call us at 310-825-6561 or email [email protected].
A member of the TRIC team will request a description of the proposed research or training and schedule a meeting to obtain further information, after which a quote will be provided.
How long does it take to start a preclinical study or training workshop once the quote is approved?
An Animal Research Committee (ARC) protocol must be completed and approved prior to work involving live animals. Our staff will work with your team to develop the appropriate ARC protocol and schedule your project as quickly as resources allow. We understand how scheduling can affect critical project timelines and will work diligently to complete your research or training as efficiently as possible.
How long does it take an ARC protocol to receive approval?
To ensure appropriate time for review by our committee, we ask that ARC protocols be submitted two to three months in advance of a study. Our staff will assist you with this process.
How long does it take to start a cadaver study or training workshop once the quote is approved ?
An Institutional Biosafety Committee (IBC) protocol must be completed and approved prior to work involving human cadavers. Additionally, an Anatomical Request form must be completed for approval to the Donated Body Program (DBP) Review Committee. Our staff will work with your team to develop the appropriate IBC protocol/DBP requests and schedule your project as quickly as resources allow. We understand how scheduling can affect critical project timelines and will work diligently to complete your research and training as efficiently as possible.
How long does it take an IBC protocol and/or DBP Anatomical Request to receive approval?
To ensure appropriate time for review by our committee, we ask that new IBC protocols or DBP requests be submitted two months in advance of a study. Our staff will assist you with this process.
Can I bring my own surgeon or interventionalist to perform the experimental surgery required as part of the study?
We welcome this level of collaboration in these studies. However, written qualifications, a CV and Medical Health Questionnaire Waiver must be provided to TRIC management in advance of the project for approval.
Can our engineers or clinical staff participate in the study?
We welcome individuals with appropriate training and experience to participate in studies. However, written qualifications, a CV and Medical Health Questionnaire Waiver must be provided to TRIC management in advance of the project for approval.
Does UCLA perform good laboratory practices (GLP) preclinical studies?
We are working diligently to provide this service in the near future.
Contact us
For more information about the UCLA Translational Research Imaging Center, please call 310-825-6561 or email us.