Neurotology and Temporal Bone Laboratories
Our laboratories are dedicated to studying the anatomy, pathology and cellular and molecular biology of the human inner ear. We use a multidisciplinary approach that includes the use of classical anatomical techniques as well as modern cellular and molecular biological methods: immunocytochemistry, unbiased quantitative stereology, transmission electron microscopy, real-time RT-PCR (for gene expression), and recently, proteomics.
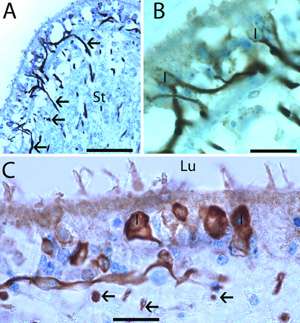
Cross-sections from the crista ampullaris (A and B) and utricular macula (C) from specimens of group 2.
(A) Specific neurofilament immunoreactivity is seen within nerve fibers throughout the crista stroma (St) (arrows). (B) A higher magnification view from the insert in (A). Immunoreactive calyceal terminals surrounding type I hair cells (I) within the sensory epithelia can be seen. (C) In the utricular macula, immunoreactive nerve fibers and calyceal terminals surrounding type I (I) hair cells can be identified. Arrows point to transversal section of immunoreactive axoplasm located in the tissue stroma. Lu: lumen. Magnification bar is (A) is 150μm, in (B) is 40μm and in (C) is 25μm.
The Microdissection Technique
To perform state-of-the-art cellular and molecular studies in the human inner ear, our laboratory has implemented the microdissection technique, which allows for rapid fixation due to the immediate removal of the encasing temporal bone, with resultant morphological preservation. Also it is possible to properly orient the different endorgans on the inner ear.
Members
- Principal Investigator: Akira Ishiyama, MD
- Collaborators: Gail Ishiyama, MD, Neurology, UCLA; Ivan A. Lopez PhD, Head and Neck Surgery, UCLA
Current Projects
- Cellular and Molecular biology of the normal and implanted human cochlea. We are investigating the effects of the cochlear implant (CI) in the different cell types of the human inner ear using immunohistochemistry and 3D reconstruction. We are investigating the presence of immunoreactive macrophages in the normal and implanted cochlea.
- Cellular and molecular biology of Meniere's disease. We have been investigating the effect of Meniere's disease in the vestibular sensory hair cells and supporting cells for several years. We are interested in the identification of blood labyrinthine cellular markers in the human inner ear using immunofluorescence and RNAScope techniques. We are interested in the identification of ultrastructural alterations in the microvasculature of the human macula utricle obtained from ablative surgery from patients diagnosed with Meniere's disease. For this purpose we use state of the art electron microscopic techniques and laser confocal fluorescence microscopy.
- The blood Labyrinthine Barrier of patients diagnosed with Meniere’s disease. We are extending our studies on the microvasculature using celloidin-embedding sections from temporal bones obtained from patients diagnosed with Meniere's disease. We are investigating the neurovascular unit (NVU) in the spiral ganglia neurons from Meniere’s disease patients and to compare with age match normal subjects.
- The cellular and molecular biology of supporting cells of the human and mouse macula utricle. Supporting cells are important for hair regeneration as well as for secreting otoconial proteins. To investigate the cellular organization we use laser confocal microscopy and electron microscopy, we offer opportunities for research in this area.
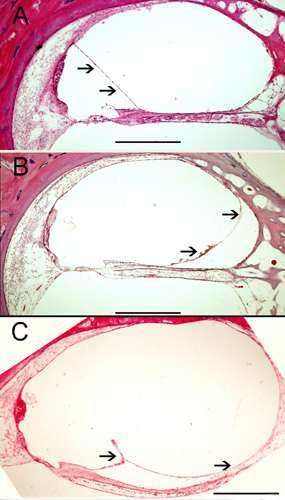
Human temporal bone celloidin embedded sections (from the UCLA temporal bone bank) stained with hematoxylin and eosin. (A) Normal view of the human cochlea (organ of Corti). Arrows point to the normal Reissner's membrane (B) and (C) shows endolymphatic hydrops from two patients diagnosed with Meniere's disease. Arrows point to the distension of the Reissner's Membrane. Magnification bar is 250 μm.
Publications
- Ishiyama A, López I, Wackym P. Subcellular innervation patterns of the calcitonin gene-related peptidergic efferent terminals in the chinchilla vestibular periphery, Otolaryngology-Head and Neck Surgery 111, 385-395, 1994.
- Ishiyama A, López I, Wackym P. Choline acetyltransferase immunoreactivity in the human vestibular end-organs, Cell Biol. Int. 18, 979-984, 1994.
- Ishiyama A, López I, Wackym P. Distribution of efferent cholinergic terminals and alfa-bungarotoxin binding to putative nicotinic acetylcholine receptors in the human vestibular periphery, Laryngoscope. 105, 1167-1172, 1995.
- Baloh RW, López I, Honrubia V, Ishiyama A, Wackym P. Vestibular Neuritis: Clinical-pathological correlation. Head and Neck-Otol. 114:586-592, 1996.
- Ishiyama A, Ishiyama G, López I, Eversole L, Honrubia V, Baloh RW. Histopathology of idiopathic chronic recurrent vertigo. Laryngoscope 106:1340-1346, 1996.
- Ishiyama A, López I, Wackym P. Molecular characterization of muscarinic receptors in the human vestibular periphery. Am J Otology, 18, 648-654, 1997.
- Baloh RW, López I, Beykirch K, Ishiyama A, Honrubia V. Clinical-pathological correlation in a patient with selective loss of hair cells in the vestibular endorgans. Neurology, 49, 1377-1382, 1997.
- Kim JS, López I, DiPatre PL, Liu F, Ishiyama, A, Baloh, RW. Internal auditory artery infarction: clinico pathologic correlation. Neurology, 52, 40-44. 1999.
- Ishiyama G, López I, Ishiyama A. Subcellular immunolocalization of NMDA receptor subunit NR-1 in the chinchilla vestibular periphery. Brain Research. 851,270-276, 1999.
- Park JJ, Tang Y, López I, Ishiyama A. Unbiased stereological quantification of neurons in the human vestibular ganglion. Neuroreport. 11,853-857, 2000.
- Lee H, López I, Ishiyama A, Baloh RW. Can migraine damage the inner ear? Arch Neurology 57, 1631-1634, 2000.
- Park J, Tang Y, López I, Ishiyama A. Age-related changes in the number of neurons in the human vestibular ganglion. J Comp Neurol 431,437-443, 2001.
- Ishiyama A, Agena J, López, I and Tang Y. Unbiased stereological quantification of neurons in the human spiral ganglion. Neuroscience Letters, 304, 93-96, 2001.
- Park J, Tang Y, López I, Ishiyama A. Unbiased estimation of human vestibular ganglion neurons. Ann NY Acad Sci, 942, 475-478, 2001.
- Tang Y, López I, Ishiyama A. Application of unbiased stereology in archival human temporal bone. Laryngoscope 112, 526-533, 2002.
- Ishiyama G, López I, Williamson R, Acuna D, Ishiyama A. Subcellular distribution of NMDA subunit NR1,2A ,2B in the rat vestibular periphery. Brain Research, 935, 16-23, 2002.
- Ishiyama G, López I, Baloh RW, Ishiyama A. Cannavan's leukodystrophy is associated with defects in cochlear neurodevelopment and deafness. Neurology, 60:1702-1704, 2003.
- Gopen Q, López I, Ishiyama G, Baloh RW and Ishiyama A. Unbiased stereological quantification of type I and type II hair cells in the human utricular macula. Laryngoscope 113:1132-1138, 2003.
- Ishiyama A. López I, Ishiyama G, Tang Y. Unbiased quantification of the microdissected human Scarpa's ganglion neurons. Laryngoscope, 114:196-1499, 2004.
- Ishiyama A, Ishiyama G, López I , Jen J, Kim G, Robert W. Baloh RW. Temporal bone histopathology in dominantly inherited audiovestibular syndrome. Neurology, 63:1859-1862, 2004.
- López I , Ishiyama G, Tang Y, Frank M, Baloh RW, Ishiyama A. Estimation of the number of nerve fibers in the human vestibular endorgans using unbiased stereology and immunohistochemistry. Journal of Neuroscience Methods, 145: 37-46, 2005.
- López I, Ishiyama G, Tang Y, Tokita J, Baloh RW, Ishiyama A. Regional estimates of hair cells and supporting cells in the human crista ampullaris. Journal of Neuroscience Research, 82:421-431, 2005.
- Ishiyama G, Finn M, López I, Tang Y, Ishiyama G. Unbiased quantification of Scarpa's ganglion neurons in aminoglycoside ototoxicity. J Vestibular Research, 15,197-202, 2005.
- Kho S, López IA, Evans C, Ishiyama A, Ishiyama G. Immunolocalization of Orphanin/FQ in Rat Cochlea. Brain Research, 113:146-152, 2006.
- Ishiyama G, López IA, Ishiyama A. Aquaporins and Meniere's disease. Curr Opin Otolaryngol Head and Neck Surg, 14:332-336, 2006.
- Ishiyama G, López IA, Ishiyama A. Histopathology of the vestibular end organs after intratympanic gentamicin failure for Meniere's disease. Acta Oto-Laryngologica, 127:34-40, 2007.
- Ishiyama G, Tokita J , López IA, , Tang Y, Ishiyama A. Unbiased stereological analysis of the human spiral ligament and stria vascularis: a temporal bone study. JARO, 8:8-17, 2007.
- López IA, Ishiyama G, Lee M, Baloh RW, Ishiyama A. Immunohistochemical localization of aquaporins in the human inner ear. Cell & Tissue Research, 328:453-460, 2007.
- Merchant SN, McKenna MJ, Adams JC, Nadol JB Jr, Fayad J, Gellibolian R, Linthicum FH Jr, Ishiyama A, López IA, Ishiyama G, Baloh R, Platt C. Human temporal bone consortium for research resource enhancement. J Assoc Res Otolaryngol, 9:1-4, 2008.
- Ishiyama A, Mowry SE, López IA Ishiyama G. Immunohistochemical distribution of basement membrane proteins in the human inner ear. Hearing Research, 2009, 254:1-14.
- McCall A, Ishiyama G, López IA, Sunita B, Ishiyama A. Histopathological and ultrastructural analysis of vestibular endorgans obtained from patients with Meniere's disease. BMC Ear Nose Throat Disord. 2009;9:4.
- Miller M, Ishiyama G, López IA, Ishiyama A. Endolymphatic Hydrops in Otologic Syphilis: A temporal bone study. Otology Neurotology, 2010, In Press.
Contact Information
Director: Akira Ishiyama, MD
Email: [email protected]
Phone: (310) 206-2041
Fax: (310) 794- 5089