UCLA Temporal Bone Laboratory
The laboratory is devoted to the microscopic study of the ears of individuals who have suffered from a hearing or balance problem and have willed their temporal bones, containing the ear structures, for scientific analysis. By comparing abnormal microscopic findings in a large number of temporal bones from individuals with similar clinical findings scientists can learn the underlying anatomical abnormality responsible for a particular disability.
Members
- Director: Akira Ishiyama MD
- Associate Director: Ivan A. Lopez, PhD, UCLA
- Laboratory Technician: Ana Cordero, UCLA
- Temporal Bone Pledge Program Coordinator: Marian Rush, UCLA
UCLA Temporal Bone Laboratory
Funding Source: NIDCD/NIH Grant U24 1U24DC015910-01
Announcement:
The UCLA Temporal Bone Laboratory in the Department of Head & Neck surgery at the "David Geffen" School of Medicine at UCLA houses a large collection of human temporal bones (2000+). Celloidin embedded human inner ear sections from this collection are freely available for basic and clinical inner ear researchers. Staining protocols and training for handling and staining this tissue is also available for staff or researchers upon request.
Ivan A. Lopez, Ph.D.
Email: [email protected]
Phone: 310-825-5331
Ongoing Projects
Our laboratory routinely performs histopathological analysis of the ears of individuals who have suffered from a hearing or balance problem and have willed their temporal bones, containing the ear structures, for scientific analysis.
By comparing abnormal microscopic findings in a large number of temporal bones from individuals with similar clinical findings scientists can learn the underlying anatomical abnormality responsible for a particular disability. The fusion of the temporal bone laboratory previously located at the House Ear Institute and the temporal bone at UCLA have been benefiting by the numerous cellular and molecular biological facilities available on UCLA campus. The daily interaction with researchers from the brain research institute and neurology allows us to implemented new methods of analysis of human temporal bones including: transmission and scanning electron microscopy to see ultrastructural changes; immunohistochemistry to identify inner ear specific proteins; in situ hybridization to locate a specific RNA or DNA sequence; 3D reconstruction to explore the contours of small structures; stereology to determine volume or surface areas; use of polarized light to observe bone structure; proteomics to characterize protein expression of inner ear tissues.
As an example of the productive interaction between both laboratories we highlight our recent review published in the journal “Histochemistry and Cell Biology”, in the review summarized the current immunohistochemical protocols and methods to study the normal and pathological human inner ear. Given the scarcity of tissue available and the high cost of processing the human temporal using the traditional methods we proposed alternative choices like microdissection, frozen and paraffin sectioning. To optimize the use of invaluable celloidin embedded sections of the human inner ear our laboratory and others, have been developing methods to remove celloidin from the sections and subsequent antigen retrieval. The best process and preserved tissue is now being shared with inner ear basic researchers to corroborate their previous finding in animal models. For this we are developing immunofluorescence protocols that allow the identification of up to 3 different proteins (See publications Lopez et al., 2016).
Our laboratory is investigating the role of melanin in the protection of the inner ear from extraneous influences such as noise, viruses, ototoxic drugs, and age. Melanin, the substance that gives skin its darker colors, is produced by special cells called melanocytes. In the skin, its acts to protect the skin cells from damage by sunlight. It is also found in other parts of the body and is believed to act as a protective substance by neutralizing the damaging effects of the products of cell metabolism called free radicals. Preliminary data indicate that there is less melanin in the base of the cochlea than in the upper segments. This may explain the increased susceptibility and resultant high frequency hearing loss, found in many conditions such as noise, viruses, age, ototoxic drugs, and heredity.

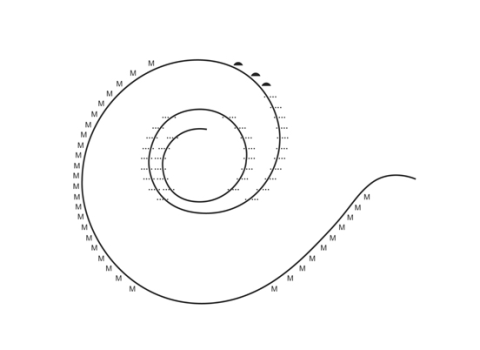
Determination of the proper correction factor for spiral ganglion counts. For many years there has some confusion among scientists doing counts of various types of cells because of "double counting". When tissue is cut in thin slices for microscopic examination, some of the cells are cut in half. It is difficult to tell if such a half cell belongs on the upper or lower slide and so might be counted twice. Using the Amira three dimensional analysis program we reconstructed a segment of the cochlear spiral ganglion filled with nerve cells. Thus, we have been able to determine the frequency that a cell, or part of a cell such as the nucleus or nucleolus, may occur (Fig 3).
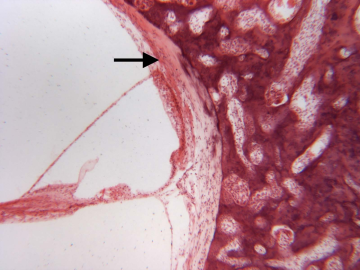
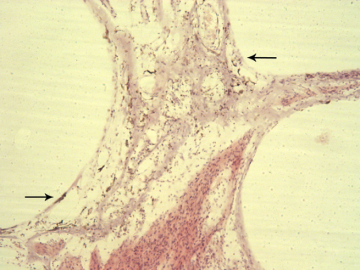
Immunohistochemical study of the cause of sensorineural hearing loss due to cochlear otosclerosis. In 1969 this laboratory reported, in the face of much controversy, for the first time a relationship of sensorineural hearing loss and the amount of otosclerosis involving the cochlear capsule. The reason for the hearing loss has yet to be determined. However in severe cases of hearing loss there are changes in the spiral ligament (hyalinization signifying aseptic cellular death) seen in no other pathological conditions affecting the inner ear. We hypothesize that the same enzymes, that are responsible for the normal bone dissolution that occurs as the otospongiosis advances through the cochlear capsule, diffuse into the spiral ligament result in cell death and replacement with hyaline and a toxic suppression of the function of the organ of Corti. We are currently investigating (using immunohistochemistry (Fig 4)) various enzymes that might be responsible for the hyalinization and cochlear malfunction.
The Temporal Bone Laboratory of the House Research Institute has moved to the campus of the University of California at Los Angeles to join UCLA's Neurotology and Temporal Bone Laboratory and is now The Neurotology and House Histological Temporal Bone Laboratory. This gives us access to some of the more advanced methods of analysis in collaboration with other departments at UCLA, such as proteomics and newer techniques of transmission electron microscopy, as are discussed below.
Histopathologic evaluation of human temporal bones began in Europe more than a century ago. It came to the United States with the establishment of the temporal bone laboratory at Johns Hopkins University by Stacey Guild, circa 1927 (Anat Rec, 1927;22:141-157). Guild developed a method of quantitative analysis of the various structures in the peripheral aspects of the cochlea (Fig. 5).
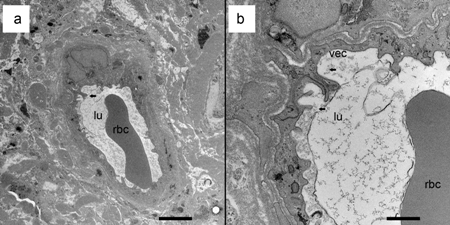
Decalcification of temporal bones using ethylenediaminetetraacetic acid (EDTA) and embedding with celloidin (nitrocellulose) for microtome sectioning is tedious and takes several months. Various acids have been tried for the decalcification to hasten the process,but due to a distortion of the organ of Corti and interference with immunohistochemical staining, they have been abandoned. Different embedding materials have also been tried, but only celloidin is able to maintain the integrity of the intracochlear anatomy because of the large fluid spaces that are not found in other types of tissue. Transmission electron microscopy has been of limited use in the study of temporal bones removed in the usual way due to subtle postmortem artifacts not visible by light microscopy. Techniques have been developed for re-embedding the celloidin sections in plastic and sectioning for transmission electron microscopy. Perfusion of the inner ear within a few hours of death can result in tissues satisfactory for transmission or scanning electron microscopy (Fig. 6).
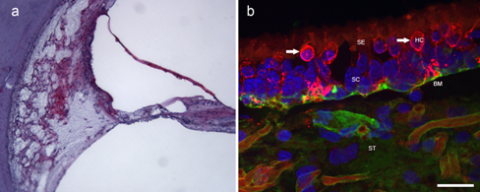
Immunohistochemistry is a method of identifying specific proteins within a cell or area. An antibody is linked to a specific chromogen or fluorescent dye and examined through a light, ultra-violet, or confocal microscope (Figs 7a and 7b) (Arnold W, Acta Otol, 1987, 436(supp):62-8.
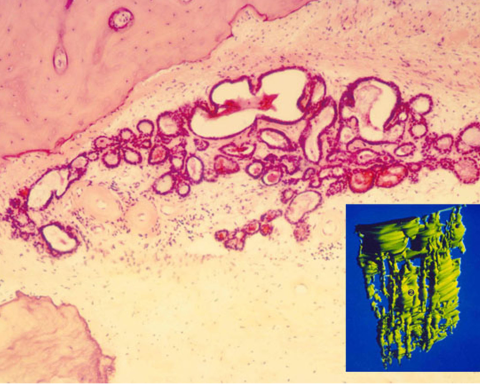
In situ hybridization has been used to locate a specific RNA or DNA sequence in a specimen (Nguyen et al, Brain Res, 2014). Three-dimensional reconstruction of microscopic inner ear anatomy has made it possible to clarify the contours of small structures that are difficult to conceptualize when seen in just two dimensions. The endolymphatic sac was thought to be a sac with a rugose surface based on animal observations. 3D reconstruction of human sacs demonstrated that the interosseous and the proximal part of the intracranial sac was actually a group of tubules connecting the cisternal part of the sac to the intracranial portion (Fig. 8) (Antunez et al, Ann Otol Rhinol Laryngol, 1980:89 (Suppl 6) 23-32). Stereology makes it possible to determine the volume or surface areas of microscopic structures such as the endolymphatic sac or cochlear structures. The measurement can be computer aided, or made with a grid overlying microphotographs or in the microscope eyepiece.
Polarized light is useful for observing the different types of bone structure in the ear (Doden and Halverm Am J Anat, 1984, 169:451-62). It is particularly effective, especially with addition of quartz light, for the examination of the different configurations of the bone structure in otosclerosis.
Proteomic studies are powerful tools that characterize the protein expression profiles of tissues, providing a platform upon which other studies may be conducted to better understand normal and disease states of specific organs. Proteomics has been applied to inner ear studies in mouse models uncovering key proteins involved in the integrity of the blood-labyrinth barrier and protein expression alterations in Usher Syndrome and cisplatin-mediated ototoxicity. Translation to human applications, however, represents a clinical dilemma. While fresh tissue remains the gold standard for tissue source, procurement of fresh human inner ear tissue has several limitations including access and limited volume. Therefore, efforts have turned toward developing techniques for proteomic analysis using human archival temporal bone specimens. These specimens are available in the temporal bone bank and may be stored indefinitely, making it the ideal alternative tissue source. Recently developed techniques have demonstrated successful celloidin removal and protein extraction, making proteomic analysis of formalin-fixed, celloidin-embedded (FFCE) human temporal bones feasible (Aarnisalo et al Hear Res, 2010:270:15-20).We have been able to detect from a mid-modiolar cochlea FFCE section from a normal control patient a total of 385 proteins, of which 252 were identified with high confidence (CI>95%). From the 252 high confidence proteins, 190 corresponded to known proteins, while 62 were unnamed proteins. Thin-sheet laser imaging microscopy (TSLIM) optical sectioning may be used to evaluate soft tissue morphology in small specimens from animals before celloidin sectioning. It has recently been used on human temporal bones (Johnson, Otol Neurotol, 2014:35:1145-9).
In summary, many new methods of analysis of human temporal bones have been added to the repertoire since the time of Guild, including electron microscopy to see greater detail; immunohistochemistry to identify proteins; in situ hybridization to locate a specific RNA or DNA sequence; 3D reconstruction to explore the contours of small structures; stereology to determine volume or surface areas; use of polarized light to observe bone structure; proteomics to characterize protein expression of inner ear tissues; and recently, TSLIM to evaluate soft tissue morphology. Undoubtedly, the future holds promise for more methods to maximize our knowledge of both normal and diseased temporal bones in order to better treat and even prevent otologic problems.
Publications
- Ishiyama G, Lopez IA, Ishiyama P, Ishiyama A. The blood labyrinthe barrier in the human normal and Meniere's macula utricle. Scientific Reports, 7(1):253. Doi:10.1038/s41598-017-00330-5, 2017.
- Ishiyama A, Doherty J, Quesnel AM, Ishiyama G, Lopez IA, Linthicum FH. Post hybrid cochlear implant hearing loss and endolymphatic hydrops. Otology & Neurotology, DOI: 10.1097/MAO.0000000000001199, 2016.
- Vorasubin N, Hosokawa S, Hosakawa K, Ishiyama G, Ishiyama A., Lopez IA. Neuroglobin immunoreactivity in the human inner ear. Brain Research, 1630: 56-63, 2016.
- Ishiyama G, Lopez IA, Sepahdari AR, Ishiyama A. Meniere's disease: histopathology, cytochemistry and imaging. Ann NY Acad Sci. 1343: 49-57, 2015.
- Nguyen KD, Mowlds D, Lopez IA, Hosokawa S, Ishiyama A, Ishiyama G. Mu-opioid receptor (MOR) expression in the human spiral ganglia. Brain Res. 1590, 10-19, 2014. PubMed PMID: 25278190.
- Ahmed S, Vorasubin N, Lopez IA, Hosokawa S, Ishiyama G, Ishiyama A. The expression of glutamate aspartate transporter (GLAST) within the human cochlea and its distribution in various patient populations. Brain Res. 2013;1529:134-42, 2013. PubMed PMID: 23850643.
- Balaker AE, Ishiyama P, Lopez IA, Ishiyama G, Ishiyama A. Immunocytochemical localization of the translocase of the outer mitochondrial membrane (Tom20) in the human cochlea. Anat Rec (Hoboken); 296(2):326-32, 2013. PubMed PMID: 23165776.
- Calzada AP, Lopez IA, Ishiyama G, Ishiyama A. Otolithic membrane damage in patients with endolymphatic hydrops and drop attacks. Otol Neurotol. 2012;33(9):1593-8.. PubMed PMID: 23064391.
- Calzada AP, Lopez IA, Beltran Parrazal L, Ishiyama A, Ishiyama G. Cochlin expression in vestibular endorgans obtained from patients with Meniere's disease. Cell Tissue Res. Nov;350(2):373-84. 2012. PubMed PMID: 22992960.
- Calzada AP, Balaker AE, Ishiyama G, Lopez IA, Ishiyama A. Temporal bone histopathology and immunoglobulin deposition in Sjogren's syndrome. Otol Neurotol. 2012 Feb;33(2):258-66. PubMed PMID: 22215450; PubMed Central PMCID: PMC3262845.
- Ishiyama G, Geiger C, Lopez IA, Ishiyama A. Spiral and vestibular ganglion estimates in archival temporal bones obtained by design based stereology and Abercrombie methods. J Neurosci Methods. 2011 Mar 15;196(1):76-80. Epub 2011 Jan 8. PubMed PMID: 21219929.
- Ishiyama G, Lopez IA, Beltran-Parrazal L, Ishiyama A. Immunohistochemical localization and mRNA expression of aquaporins in the macula utriculi of patients with Meniere's disease and acoustic neuroma. Cell Tissue Res. 2010 Jun;340(3):407-19. Epub 2010 May 12. PubMed PMID: 20461409; PubMed Central PMCID: PMC2882038.
- McCall AA, Ishiyama GP, Lopez IA, Bhuta S, Vetter S, Ishiyama A. Histopathological and ultrastructural analysis of vestibular endorgans in Meniere's disease reveals basement membrane pathology. BMC Ear Nose Throat Disord. 2009 Jun 3;9:4.. PubMed PMID: 19493357; PubMed Central PMCID: PMC2701917.
- Ishiyama A, Mowry SE, Lopez IA, Ishiyama G. Immunohistochemical distribution of basement membrane proteins in the human inner ear from older subjects. Hear Res. 2009 Aug;254(1-2):1-14. Epub 2009 Apr 5. PubMed PMID: 19348877; PubMed Central PMCID: PMC2758085.
- Lopez IA, Ishiyama G, Lee M, Baloh RW, Ishiyama A. Immunohistochemical localization of aquaporins in the human inner ear. Cell Tissue Res. 2007 Jun;328(3):453-60. Epub 2007 Feb 21. PubMed PMID: 17318586.
- Linthicum, F.H., Jr.: "The Effect of Surgical Trauma in Otosclerosis". Laryngoscope, 75:35, January, 1965.
- Kelemen, G. and Linthicum, F.H., Jr.: "Otosclerotic Focus and Facial Canal". Arch. Otolaryngol., 82:575-578, December, 1965
- Linthicum, F.H., Jr.: "Correlation of Sensorineural Hearing Impairment and Otosclerosis". Ann. Otol. Rhinol. Laryngol. 75:512-524, June, 1966.
- Linthicum, F.H., Jr. and Sheehy, J.L: "Blood in the Vestibule at Stapedectomy; Human Case Report with Histological Findings". Ann. Otol. Rhiol. Laryngol., 78:425-429, April, 1969.
- Kelemen, G. and Linthicum, F.H., Jr.: "Labyrinthine Otosclerosis". Acta Oto-Laryngologica. Supp. 253, 1969.
Britton, B.H., Jr., and Linthicum, F.H., Jr.: "Otosclerosis Histologic Confirmation of Radiologic Findings". Ann. Otol. Rhinol., 79:1, February 1970. - Linthicum, F.H., Jr.: "Diagnosis of Cochlear Otosclerosis". Arch. Otolaryngol., 95:564-469, June, 1972.
- Linthicum, F.H., Jr.; House, H.P. and Althaus, S.A.: "The Effect of Sodium Fluoride on Otosclerotic Activity as Determined by Strontium85". Ann. Otol. Rhinol. Laryngol, 82:6099, 1973.House, H.P. and Linthicum, F.H., Jr.: "Sodium Fluoride and the Otosclerotic Lesion". Arch. Otolaryngol., 100:427-430, 1974.
- Ghorayeb, B.Y and Linthicum, F.H., Jr.: "Otosclerotic Inner Ear Syndrome". Ann. Otol. Rhinol. Laryngol, 87:85-90, 1978.
- "The Effect of Stapedectomy on Hearing of Patients with Otosclerosis and Meniere's Disease". Am. J. Otolaryngol., 4(4):323-336, April, 1983.
- Parahy, C. and Linthicum, F.H., Jr.: "Otosclerosis: Relationship of Spiral Ligament Hyalinization to Sensorineural Hearing Loss". Laryngoscope, 93(6):717-720, June, 1983
- Parahy, C. and Linthicum, F.H., Jr.: "Otosclerosis and Otospongiosis: Clinical and Histological Comparisons". Laryngoscope, 94(4):508-512, April, 1984.
- Linthicum, F.H., Jr.: "Cochlear Otosclerosis". Current Therapy in Otolaryngology-Head and Neck Surgery, 1984-1985, pg 37, B.C. Decker, Inc., and C.V. Mosby Company, Publishers. St. Louise, Missouri.
- Linthicum, F.H., Jr. and Forquer, Briand D.: "Sodium Fluoride as a Treatment for Otosclerotic Hearing Loss". Am. J. Otol. 6:1, pg 35-37, January, 1985
- Forquer, B.D.; Linthicum, F.H., Jr.; Bennett, C.: "Sodium Fluoride: Effectiveness of Treatment for Cochlear Otosclerosis". Am. J. Otol., 7(2):121-125, March, 1986.
- Fayad, J.; Moloy, P.; Linthicum, F.H., Jr.: "Cochlear Otosclerosis: Does Bone Formation Affect Cochlear Implant Surgery?" Am. J. Otol. 11(3)96-200, May 1990.
- D'Ascanio L, Linthicum FH Jr. Multiple Otosclerotic Foci Without Hearing Loss.Otol Neurotol. 2006 Dec
- Xenellis JE, Linthicum FH Jr. Malleus head fixation with stapedial otosclerosis. Otol Neurotol.
Please feel free to request tissue sections for research purpose to:
Ivan A Lopez PhD
Adjunct Professor
Associate Director of the UCLA National Temporal Bone Laboratory
Department of Head & Neck Surgery
David Geffen School of Medicine at UCLA
1000 Veteran Avenue
Room 35-64, Rehabilitation Center
Los Angeles, CA 90095
Phone: 310-825-5331
Email: [email protected]
Email: [email protected]