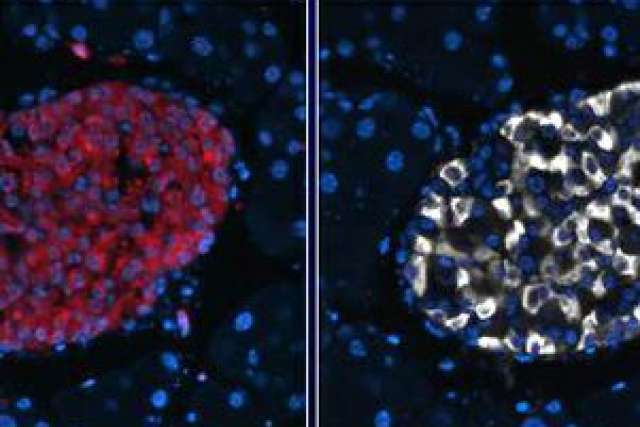
People with Type 2 diabetes have an excess of a protein called islet amyloid polypeptide, or IAPP, and the accumulation of this protein is linked to the loss of insulin-producing pancreatic beta cells.
What causes this accumulation of IAPP in pancreatic beta cells of people with diabetes has remained a mystery. But a team of researchers from the Larry L. Hillblom Islet Research Center led by Dr. Peter Butler, professor of medicine at UCLA, may have found an answer in autophagy, a process that clears damaged and toxic proteins from cell.
In a study published online July 18 in the peer-reviewed Journal of Clinical Investigation, the UCLA researchers suggest that, in people who do not have Type 2 diabetes, autophagy prevents the accumulation of toxic forms of IAPP. In people with Type 2 diabetes, the process appears to not work properly, contributing to the destruction of beta cells. As the body’s insulin producers, beta cells play a key role in maintaining healthy blood sugar levels.
"Only a few previous studies have reported that autophagy is important for beta cell function and survival," said Safia Costes, a research scientist at the Hillblom Center and the study's co-first author. "Those studies, however, were not conducted to address the role of this process in the regulation of the amyloidogenic protein, which is an important contributor to Type 2 diabetes."
Investigators found that autophagy plays a role in clearing IAPP from pancreatic beta cells using three experimental models: pancreatic beta cells, isolated pancreatic islets from mice that express the human form of IAPP, and human islets.
To corroborate the findings, the researchers also developed a novel mouse model that was deficient for autophagy specifically in beta cells with expression of the human form of islet amyloid polypeptide. They found that mice that had beta cells in which autophagy didn't work properly showed elevated levels of toxic IAPP, which led to the death of the beta cells. As a result, those mice developed diabetes.
"The goal of our work is to understand the cellular mechanisms responsible for beta cell destruction so that we can identify the best targets for beta cell protection," Costes said. "This would aid the development of the next generation of treatments as well as combination therapies for Type 2 diabetes."
The study also confirmed similarities between Type 2 diabetes and Alzheimer's and other neurodegenerative diseases that are marked by an accumulation of toxic forms of amyloid proteins, she said. "This demonstrates the importance of autophagy in clearing out these harmful proteins to prevent both Type 2 diabetes and Alzheimer's."
The study's other co-first author was Jacqueline Rivera, a postdoctoral fellow at the Hillblom Center. Butler was the senior author. Other authors were Tatyana Gurlo from UCLA and Charles Glabe from UC Irvine.
The research was supported by grants from National Institute of Diabetes and Digestive and Kidney Diseases (DK059579 and DK090995), NIH (AG033069), the Larry L. Hillblom Foundation (2012-D-001-SUP and 2007-D-003-NET), and the Esther B. O'Keeffe Foundation.