Yalda Afshar MD PhD Lab

Research Lab of Yalda Afshar, MD, PhD
Additional information on the Afshar Lab

UCLA Perinatal Biospecimen Repository
Research participants needed to join the effort to improve maternal and child health.
Afshar Laboratory
congenital heart disease – cardio-obstetrics – placentation
The Afshar lab is a translational laboratory integrating cutting edge prenatal maternal-fetal imaging, underlying genetic predispositions, and environmental clues to understand congenital heart disease and the placenta-cardiac axis. The laboratory seeks to understand the upstream and downstream signaling alterations in congenital heart disease and the placenta to define what we have termed the prenatal vascular phenotype.
Laboratory Research
The Prenatal Vascular Phenotype and Fetal Congenital Heart Disease
Congenital heart defects (CHDs) are the most common cause of congenital anomaly, occurring in ~1% of newborns. These neonates generally survive in-utero; however, there is lifetime morbidity related to vascular sequalae. The etiology(ies) of CHD remain elusive making intervention purely mechanical. Upstream and/or downstream signaling alterations in endothelial mechano-transduction throughout their life due to the abnormal flow patterns, remains a unifying phenotype. We have termed this the prenatal vascular phenotype.
To understand and target the mechanisms underlying the vascular phenotype, we aim to integrate data through the life course -- the prenatal and postnatal sequalae. We couple prenatal maternal-fetal imaging, underlying genetic predispositions, and developmental endothelial flow dynamics. Ultimately, this may improve treatment that arise from loss of vascular integrity. Despite being born with a morbid CHD, we have a chance to understand downstream complications and interventions that can improve quality of life in these patients with lifelong sequelae related to an abnormal endothelial cell and vascular phenotype.
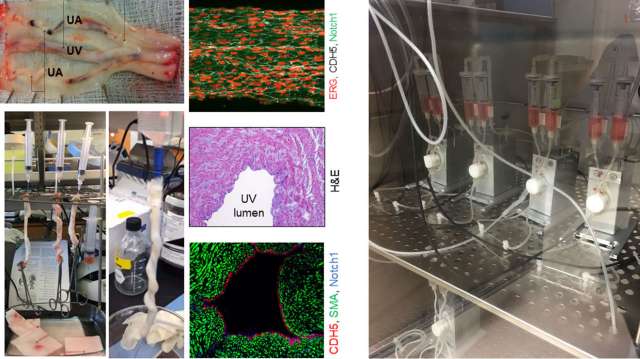
Related publications:
- Afshar Y, Hogan WJ, Conturie C, Sunderji S, Duffy JY, Peyvandi S, Boe NM, Melber D, Fajardo VM, Tandel MD, Holliman K, Kwan L, Satou G, Moon-Grady AJ. Multi-Institutional Practice-Patterns in Fetal Congenital Heart Disease Following Implementation of a Standardized Clinical Assessment and Management Plan. J Am Heart Assoc. 2021 Aug 3;10(15):e021598. doi: 10.1161/JAHA.121.021598. Epub 2021 Jul 28. PMID: 34315235.
- Imany-Shakibai H, Yin O, Russell MR, Sklansky M, Satou G, Afshar Y. Discordant congenital heart defects in monochorionic twins: Risk factors and proposed pathophysiology. PLoS One. 2021 May 6;16(5):e0251160. doi: 10.1371/journal.pone.0251160. PMID: 33956871; PMCID: PMC8101911.
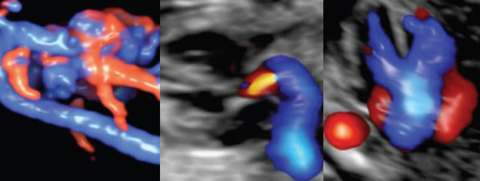
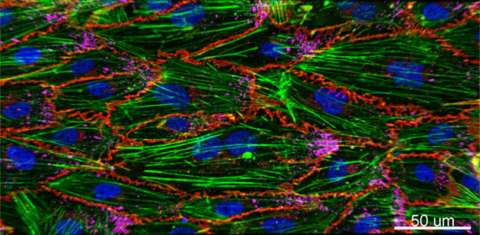
Cardio-Obstetrics and Maternal Congenital Heart Disease
Significant advancements in medical care have allowed women with maternal congenital heart disease (CHD) to survive to childbearing age, resulting in an increased prevalence of cardiovascular disease in pregnancy. Although pregnancy and its associated hemodynamic changes may be well tolerated in some, pregnancy may increase the risk of volume overload, development of arrhythmias, and progressive cardiac dysfunction in others. The presence of maternal CHD is also a major determinant for neonatal morbidity. Overall, pregnancies complicated by maternal CHD are at an increased risk for adverse maternal and neonatal outcomes compared to the general obstetric population. Our translational team focuses on optimizing both delivery coordination and planning for this high-risk population as well as understanding molecular mechanisms that underlie normal and abnormal physiological changes of the cardiovascular system in pregnancy. Clinical Cardio-OB program
Related Publications:
- Afshar Y, Tan W, Jones WM, Canobbio M, Lin J, Reardon L, Lluri G, Aboulhosn J, Koos BJ. Maternal Fontan procedure is a predictor of a small-for-gestational-age neonate: a 10-year retrospective study. Am J Obstet Gynecol MFM. 2019 Aug;1(3):100036. doi: 10.1016/j.ajogmf.2019.100036. Epub 2019 Aug 30. PMID: 33345800.
- Yin O, Woods A, Koos B, DeVore GD, Afshar Y. Central hemodynamics are associated with fetal outcomes in pregnancies of advanced maternal age. Pregnancy Hypertension, 2020 Jan;19:67-73. doi: 10.1016/j.preghy.2019.12.009. PMID: 31923879.
- Woods A, Afshar Y, Yin O, Jones WM, Kwan L, Zhang H, Koos BJ, DeVore G. Maternal Central Blood Pressure is Associated with Fetal Middle Cerebral Artery Dopplers. Reproductive Sciences. 2020 Feb; 27(2): 655-661. Doi:10.1007/s43032-019-00069-6. PMID: 32046428.
The Placenta in Health and Disease
Our lab focuses on normal and abnormal placentation in pregnancy. We’re interested in the placenta as a transient support organ in fetal development, in cardiac development, as well as abnormal placentation, as in placenta accreta spectrum disorders and preeclampsia.
Placenta accreta spectrum (PAS) disorders are potentially life-threatening pregnancy condition that occurs when the placenta invades into the uterus. The deeper the invasion of the placenta into the uterine muscle, the more severe the PAS.In most pregnancies, the placenta separates from the uterus after birth, and is delivered as the ‘afterbirth’. When the placenta cannot detach, life-threatening hemorrhage may occur. Early detection is lifesaving: care coordination plans are enacted, including delivery at a specialized care center to minimize risk to the women and her baby, including the ability of uterine preservation.
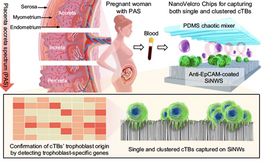
Related Publications:
- Afshar Y, Dong J, Zhao P, Li L, Wang S, Zhang RY, Zhang C, Yin O, Han CS, Einerson BD, Gonzalez TL, Zhang H, Zhou A, Yang Z, Chou SJ, Sun N, Cheng J, Zhu H, Wang J, Zhang TX, Lee YT, Wang JJ, Teng PC, Yang P, Qi D, Zhao M, Sim MS, Zhe R, Goldstein JD, Williams J 3rd, Wang X, Zhang Q, Platt LD, Zou C, Pisarska MD, Tseng HR, Zhu Y. Circulating trophoblast cell clusters for early detection of placenta accreta spectrum disorders. Nat Commun. 2021 Aug 3;12(1):4408. doi: 10.1038/s41467-021-24627-2. PMID: 34344888; PMCID: PMC8333096.
- Gonzalez TL, Eisman LE, Joshi N, Flowers AE, Wu D, Wang Y, Santiskulvong C, Tang J, Buttle RA, Sauro E, Clark EL, DiPentino R, Jeffries CA, Chan JL, Lin Y, Zhu Y, Afshar Y, Tseng HR, Taylor K, Williams J, Pisarska MD. High-throughput miRNA-sequencing of the human placenta: expression throughout gestation. 2021 May 25. doi: 10.2217/epi-2021-0055. Epub ahead of print. PMID: 34030457.
- Fratto, V, Conturie, C, Ballas, J, Pettit, K, Stephenson, Megan L, Truong, Yen N, Henry, D, Afshar Y, Murphy A, Kim, L, Field , N, Wing D, Norton ME, Ramos GA, Fetal Consortium, University of California. Assessing the multidisciplinary team approaches to placenta accreta spectrum across five institutions within the University of California Fetal Consortium (UCfC). Journal of Maternal Fetal and Neonatal Medicine. 2019 Oct 24:1-6. doi: 10.1080/14767058.2019.1676411. PMID: 31645153.
Solid Organ Transplantation and Pregnancy (the fetal allograft)
Recipients of solid organ transplants who become pregnant represent an obstetrically high-risk population. Preconception planning and effective contraception tailored to the individual patient are critical in this group. Planned pregnancies improve both maternal and neonatal outcomes and provide a window of opportunity to mitigate risk and improve lifelong health. Optimal management of these pregnancies is not well defined. Common pregnancy complications after transplantation include hypertension, preterm birth, infection, and metabolic disease. Multidisciplinary preconception and prepartum management, and counseling decrease complications and benefit the maternal-neonatal dyad. We focus on understanding the fetal allograft in the setting of solid-organ transplantation and pregnancy.
Related Publications:
- Yin O, Kallapur A, Coscia L, Constantinescu S, Moritz M, Afshar Y. Differentiating Acute Rejection From Preeclampsia After Kidney Transplantation. Obstet Gynecol. 2021 Jun 1;137(6):1023-1031. doi: 10.1097/AOG.0000000000004389. PMID: 33957644; PMCID: PMC8141043.
- Yin O, Kallapur A, Coscia L, Constantinescu S, Moritz M, Afshar Y. Obstetric and graft outcomes based on mode of delivery in kidney and liver transplant recipients: a report from the National Transplantation Pregnancy Registry. JAMA Network Open. In Press.
- Kallapur A, Jang C, Yin O, Mei JY, Afshar Y. Pregnancy care in solid organ transplant recipients. Int J Gynaecol Obstet. 2021 Jul 10. doi: 10.1002/ijgo.13819. Epub ahead of print. PMID: 34245162.
COVID-19 and Pregnancy, PRIORITY (Pregnancy CoRonavirus Outcomes RegIsTrY) and COVID-19

PRIORITY (Pregnancy CoRonavIrus Outcomes RegIsTrY), a nationwide registry of pregnant and postpartum women with or known or suspected COVID-19. Our team at UCLA has partnered with UCSF to establish this collaboration to answer critical questions for peripartum, neonatal, and postpartum patients with COVID-19 nationally and globally. Little is known about COVID-19 and its longstanding impact for women and babies. A robust research platform is needed to better understand the pathways of COVID-19 to identify risk factors and develop effective treatments. Our work includes maternal and neonatal outcomes and a biospecimen core to answer clinically relevant questions.
For more information about the study
Donation page
Related Publications:
- Afshar Y, Gaw S, Flaherman V, Chambers B, Krakow D, Berghella V, Shamshirsaz A, Aldrovandi G, Greiner A, Riley L, Boscardin WJ, Jamieson D, Jacoby V. Clinical Presentation of Coronavirus 2019 (CoVID-19) in Pregnant and Recently Pregnant People. Obstetrics and Gynecology, 2020 Oct 7. doi: 10.1097/AOG.0000000000004178. Epub ahead of print. PMID: 33027186.
- Flaherman VJ, Afshar Y, Boscardin J, Keller RL, Mardy An, Prahl MK, Phillips C, Asiodu IV, Berghella V, Chambers BD, Crear-Perry J, Jamieson Dm, Jacoby VL, Gaw SL. Infant Outcomes Following Maternal Infection with SARS-CoV-2: First Report from the PRIOIRTY study. Clinical Infectious Disease. 2020 Sept 18: doi. 10.1093/cid/ciaa1411. Online ahead of print. PMID: 32947612
- Douglass KM, Strobel KM, Richley M, Mok T, de St Maurice A, Fajardo V, Young AT, Rao R, Lee L, Benharash P, Chu A, Afshar Y. Maternal-Neonatal Dyad Outcomes of Maternal COVID-19 Requiring Extracorporeal Membrane Support: A Case Series. Am J Perinatol. 2020 Oct 17. doi: 10.1055/s-0040-1718694. Epub ahead of print. PMID: 33069171.
- Afshar Y, Parchem JG. Pregnant during the pandemic: United in motherhood. EClinicalMedicine. 2021 Feb 24;33:100760. doi: 10.1016/j.eclinm.2021.100760. PMID: 33681746; PMCID: PMC7918252.
Publications
PubMed
Google Scholar
Division of Maternal Fetal Medicine Research and Publications
Additional Publications