The Cannesson Laboratory
Proteomics and genomics can only go so far in predicting cardiorespiratory instability and managing critically ill patients. The missing link is phenomics: the study of the interaction of genes and the environment, which can help us characterize disease processes and the determinants that make an individual more or less susceptible to them. The use of phenomics can leverage the proteomics and genomics of patients to operationalize frameworks suitable for effective clinical decision-making, thus creating truly precise precision medicine.
Our laboratory is focusing on the phenomics of impending physiologic instability in patients undergoing surgery. Our goal is to develop, validate, and test real-time risk prediction tools based on electronic health record (EHR) data and high-fidelity physiological waveforms to predict physiological instability in surgical patients. This is extremely relevant because 5.7 million patients in the U.S. are admitted to an intensive care unit each year, and more than 42 million undergo surgery annually.
Our approach is based on the following expertise:
- Development and validation of novel monitoring systems with a focus on hemodynamic and cardiovascular monitoring. Over the years we have pioneered the development and clinical validation of non-invasive monitoring solutions in the perioperative setting. Specifically, our expertise focuses on the concept of functional hemodynamic monitoring.
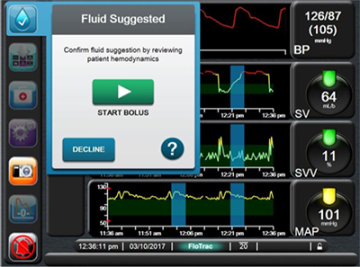
- Development of clinical decision support and closed loop anesthesia systems. We have led the development and validation of hemodynamic closed loop management systems and clinical decision systems, based on fluid management and vasopressor management in the critical care setting and in the surgical setting. Some of these systems are commercially available in Europe (Assisted Fluid Management system – Figure 1) and are used by clinicians at the bedside to make decisions during surgery.
- Development of predictive physiological tools using EHR and high-fidelity waveform data based on feature-extraction techniques and machine learning approaches (Figure 2).
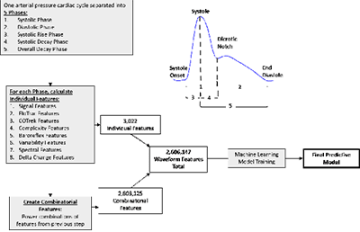
Our work involves in silico development and validation of software that we then test in the simulation setting and in the clinical setting. We collaborate with other investigators at UCLA, UC Irvine, the University of Pittsburgh Medical Center, and Carnegie Mellon University.
We leverage our previous work with NIH/R01 and industry-funded projects in the intensive care unit and surgical settings to characterize physiological instability during surgery. Our ongoing work includes:
- Improving surgical practice by early and accurate identification of the patients who are at greatest risk for physiological instability, and by providing data-driven explanations and process-specific resuscitation guidance using real-time data input and point-of-care management, potentially decreasing preventable surgical morbidity and mortality.
- Being able to adjust for placement, level of monitoring needed, and preemptive therapies and response to therapy, enabling personalized medicine. The goal is to create a sensitive and specific means to predict which patients may or may not ever develop physiological instability, which has important implications for patient safety, surveillance, triage, and care: needed frequency of monitoring, case load mixture, workload, staff allocation, patient triage to monitored or non-monitored units, higher cost vs. lower cost bed allocation, and prevention of adverse events.
- Few innovations have been introduced in recent decades that can materially improve technologic patient surveillance and guide clinical management. The modeling methods we develop should be able to shift intraoperative paradigms and treatment protocols significantly, and will be applicable to existing monitoring modalities.
- Our machine-learning analytics have the potential to identify new monitoring parameters to improve the prediction of instability and, in our exploratory specific aim, to reverse-engineer our understanding of physiology and disease during surgery.
CLINICAL INNOVATIONS
In this section:
- Development and Validation of Novel Hemodynamic Monitoring Systems
- New Care Delivery Models
- Closed Loop and Clinical Decision Support Systems
- Health Informatics and Physiological Predictive Tools
Development and Validation of Novel Hemodynamic Monitoring Systems
We have developed and introduced into clinical practice non-invasive hemodynamic monitoring systems that include utilization of the respiratory variations in the pulse oximeter waveform to predict fluid responsiveness and guide fluid management. The technology has been integrated as part of multiple monitoring systems and widely adopted internationally (under the name Pleth Variability IndexTM).
Significant Publications:
- Cannesson M, Besnard C, Durand PG, Bohe J, Jacques D. Relation between respiratory variations in pulse oximetry plethysmographic waveform amplitude and arterial pulse pressure in ventilated patients. Crit Care. 2005;9:R562-R568.
- Cannesson M, Attof Y, Rosamel P, *Desebbe O, Joseph P, Metton O, Bastien O, Lehot JJ. Respiratory Variations in Pulse Oximetry Plethysmographic Waveform Amplitude to Predict Fluid Responsiveness in the Operating Room. Anesthesiology 2007;106:1105-1111.
- Cannesson M, *Slieker J, Desebbe O, Bauer C, Chiari P, Hénaine R, Lehot JJ. Ability of a novel algorithm for automatic estimation of the respiratory variations in arterial pulse pressure to monitor fluid responsiveness in the operating room. Anesth Analg 2008;106:1195-2000.
- Cannesson M, Le Manach Y, Hofer CK, Goarin JP, Lehot JJ, Vallet B, Tavernier B. Assessing the Diagnostic Accuracy of Pulse Pressure Variations for the Prediction of Fluid Responsiveness: a “Gray Zone” Approach. Anesthesiology 2011; 115:231-41.
- Cannesson M, *Tran NP, Cho M, Hatib F, Michard F. Predicting Fluid Responsiveness with Stroke Volume Variation despite Multiple Extrasystoles. Crit Care Med 2012; 40: 193-8.
- Cannesson M, Jian Z, *Chen G, Vu TQ, Hatib F. Effects of phenylephrine on cardiac output and venous return depend on the position of the heart on the Frank-Starling relationship. J Appl Physiol. 2012;113:281-9.
- *Desebbe O, *Joosten A, *Suehiro K, Lahham S, Essiet M, Rinehart J, Cannesson M. A Novel Mobile Phone Application for Pulse Pressure Variation Monitoring Based on Feature Extraction Technology: A Method Comparison Study in a Simulated Environment. Anesth Analg. 2016 Jul;123(1):105-13.
- Barjaktarevic I, Toppen WE, Hu S, Aquije Montoya E, Ong S, Buhr R, David IJ, Wang T, Rezayat T, Chang SY, Elashoff D, Markovic D, Berlin D, Cannesson M. Ultrasound Assessment of the Change in Carotid Corrected Flow Time in Fluid Responsiveness in Undifferentiated Shock. Crit Care Med. 2018 Aug 21. doi: 10.1097/CCM.0000000000003356. [Epub ahead of print]
New Care Delivery Models: The interplay between new medical technology implementation and healthcare delivery
This research studies the implementation and dissemination of new models of perioperative healthcare delivery systems and the impact on healthcare delivery. Our group has been involved in the implementation of new models of perioperative care such as the Perioperative Surgical Home and Enhanced Recovery After Surgery. We have led the development and growth of the preoperative clinic, the implementation of telehealth for perioperative visits, implementation of digital quality improvement projects to improve quality of care and patient outcome using clinical decision supports embedded in the EHR, and implementation of Enhanced Recovery After Surgery pathways for multiple surgical lines. All these perioperative medicine initiatives have been developed in collaboration with surgical services, nursing, IT, and administration.
Significant Publications:
- Kain ZN, Vakharia S, Garson L, Engwall S, Schwarzkopf R, Gupta R, Cannesson M. The perioperative surgical home as a future perioperative practice model. Anesth Analg 2014; 118: 1126-30
- Garson L, Schwarzkopf R, Vakharia S, *Alexander B, Stead S, Cannesson M, Kain ZN. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg 2014; 118:1081-9
- Raphael DR, Cannesson M, Rinehart J, Kain ZN. Health Care Costs and the Perioperative Surgical Home: A Survey Study. Anesth Analg. 2015 Jul 28. [Epub ahead of print]
- Cyriac J, Garson L, Schwarzkopf R, Ahn K, Rinehart J, Vakharia S, Cannesson M, Kain Z. Total Joint Replacement Perioperative Surgical Home Program: 2-Year Follow-Up. Anesth Analg. 2016;123(1):51-62.
- Qiu C, Cannesson M, Morkos A, Nguyen VT, LaPlace D, Trivedi NS, Khachatourians A, Rinehart J, Kain ZN. Practice and Outcomes of the Perioperative Surgical Home in a California Integrated Delivery System. Anesth Analg. 2016;123(3):597-606.
- Desebbe O, Lanz T, Kain Z, Cannesson M. The perioperative surgical home: An innovative, patient-centered and cost-effective perioperative care model. Anaesth Crit Care Pain Med. 2016;35:59-66.
- Brandal D, Keller MS, Lee C, Grogan T, Fujimoto Y, Gricourt Y, Yamada T, Rahman S, Hofer I, Kazanjian K, Sack J, Mahajan A, Lin A, Cannesson M. Impact of Enhanced Recovery After Surgery and Opioid-Free Anesthesia on Opioid Prescriptions at Discharge From the Hospital: A Historical-Prospective Study. Anesth Analg. 2017 Nov;125(5):1784-1792.
- Soffin EM, Gibbons MM, Ko CY, Kates SL, Wick EC, Cannesson M, Scott MJ, Wu CL. Evidence Review Conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery: Focus on Anesthesiology for Total Hip Arthroplasty. Anesth Analg. 2018 Jul 21. doi: 10.1213/ANE.0000000000003663. [Epub ahead of print]
- Grant MC, Gibbons MM, Ko CY, Wick EC, Cannesson M, Scott MJ, McEvoy MD, King AB, Wu CL. Evidence Review Conducted for the Agency for Healthcare Research and Quality Safety Program for Improving Surgical Care and Recovery: Focus on Anesthesiology for Bariatric Surgery. Anesth Analg. 2018 Aug 14. doi: 10.1213/ANE.0000000000003696. [Epub ahead of print]
Closed Loop and Clinical Decision Support Systems
After in silico, in vivo and clinical studies, these closed-loop (automated) hemodynamic management systems are now tested at multiple institutions internationally. The fluid management system has been licensed to Edwards Lifesciences, and has been commercialized in Europe since March 2017 (Figure 1). This work is currently supported by the NIH under one U54 mechanism (Award Number U54HL119893, NIH/NCATS UL1-TR0001414) and an industry partnership.
Significant Publications:
- Rinehart J, Liu N, Alexander B, Cannesson M. Review article: closed-loop systems in anesthesia: is there a potential for closed-loop fluid management and hemodynamic optimization? Anesth Analg. 2012 Jan;114(1):130-43.
- Rinehart J, Alexander B, Le Manach Y, Hofer C, Tavernier B, Kain ZN, Cannesson M. Evaluation of a novel closed-loop fluid administration system based on dynamic predictors of fluid responsiveness: an in-silico simulation study. Crit Care. 2011 Nov 23;15(6):R278.
- Rinehart J, Lilot M, Lee C, Joosten A, Huynh T, Canales C, Imagawa D, Demirjian A, Cannesson M. Closed Loop Assisted vs. Manual Goal-Directed Fluid Therapy During High-Risk Abdominal Surgery: A Case Control Study with Propensity Matching. Crit Care 2015; 19;19:94.
- Rinehart J, Lee C, Canales C, Kong A, Kain Z, Cannesson M. Closed-loop fluid administration compared to Anesthesiologist management for hemodynamic optimization and resuscitation during surgery: an in vivo study. Anesth Analg 2013; 117:1119-1129
- Rinehart J, Lee C, Cannesson M, Dumont G. Closed-loop fluid resuscitation: robustness against weight and cardiac contractility variations. Anesth Analg 2013; 117: 1110-1118
- Nair BG, Gabel E, Hofer I, Schwid HA, Cannesson M. Intraoperative Clinical Decision Support for Anesthesia: A Narrative Review of Available Systems. Anesth Analg. 2017;124(2):603-617.
- Joosten A, Jame V, Alexander B, Chazot T, Liu N, Cannesson M, Rinehart J, Barvais L. Feasibility of Fully Automated Hypnosis, Analgesia, and Fluid Management Using 2 Independent Closed-Loop Systems During Major Vascular Surgery: A Pilot Study. Anesth Analg. 2018 May 9. doi: 10.1213/ANE.0000000000003433. [Epub ahead of print]
- Joosten A, Raj Lawrence S, Colesnicenco A, Coeckelenbergh S, Vincent JL, Van der Linden P, Cannesson M, Rinehart J. Personalized Versus Protocolized Fluid Management Using Noninvasive Hemodynamic Monitoring (Clearsight System) in Patients Undergoing Moderate-Risk Abdominal Surgery. Anesth Analg. 2018 Jun 5. doi: 10.1213/ANE.0000000000003553. [Epub ahead of print]
- Joosten A, Delaporte A, Ickx B, Touihri K, Stany I, Barvais L, Van Obbergh L, Loi P, Rinehart J, Cannesson M, Van der Linden P. Crystalloid versus Colloid for Intraoperative Goal-directed Fluid Therapy Using a Closed-loop System: A Randomized, Double-blinded, Controlled Trial in Major Abdominal Surgery. Anesthesiology, 2018 Jan;128(1):55-66
Health Informatics and Physiological Predictive Tools
Our research group specializes in the development of predictive and prescriptive tools based on machine-learning techniques to predict clinical outcome in the perioperative setting. Our expertise focuses on complex analysis of physiologic signals using computer science techniques to develop predictive tools. In parallel to the development of clinical tools to help clinicians forecast vital signs during surgery, this approach also aims at reverse-engineering the statistical associations found between physiological waveform features and clinical outcome in order to augment our understanding of human physiology. This work is currently supported by the NIH under three R01 mechanisms (two sub awards R01-GM117622, R01-NR013912, and one pending R01-HL144692) and an industry partnership.
Significant Publications:
- Cannesson M, Tanabe M, Suffoletto MS, McNamara DD, Madan S, Lacomis LM, Gorcsan J. A novel two-dimensional echocardiographic image analysis system using artificial intelligence learned pattern recognition for rapid automated ejection fraction. J Am Coll Cardio 2007;49:217-26.
- Gabel E, Hofer IS, Satou N, Grogan T, Shemin R, Mahajan A, Cannesson M. Creation and Validation of an Automated Algorithm to Determine Postoperative Ventilator Requirements After Cardiac Surgery. Anesth Analg. 2017 May;124(5):1423-1430.
- Hofer IS, Cheng D, Grogan T, Fujimoto Y, Yamada T, Beck L, Cannesson M, Mahajan A. Automated Assessment of Existing Patient's Revised Cardiac Risk Index Using Algorithmic Software. Anesth Analg. 2018 May 25. doi: 10.1213/ANE.0000000000003440. [Epub ahead of print]
- Hofer IS, Halperin E, Cannesson M. Opening the Black Box: Understanding the Science Behind Big Data and Predictive Analytics. Anesth Analg. 2018 May 25. doi: 10.1213/ANE.0000000000003463. [Epub ahead of print]
- Lee CK, Hofer I, Gabel E, Baldi P, Cannesson M. Development and Validation of a Deep Neural Network Model for Prediction of Postoperative In-hospital Mortality. Anesthesiology. 2018 Apr 17. doi: 10.1097/ALN.0000000000002186. [Epub ahead of print]
- Hatib F, Jian Z, Buddi S, Lee C, Settels J, Sibert K, Rinehart J, Cannesson M. Machine-learning Algorithm to Predict Hypotension Based on High-fidelity Arterial Pressure Waveform Analysis. Anesthesiology. 2018 Jun 12. doi: 10.1097/ALN.0000000000002300. [Epub ahead of print]