High Risk Patient Population (Who is high risk for breast cancer?)
by Shannon Yoo MD and Najmi Nooshin MD
The current guidelines per the American College of Radiology (ACR) recommend annual screening mammogram beginning at the age of 40 for women with an average risk of breast cancer. The individuals assessed to be at higher than average risk should be on an alternative screening schedule, which typically begins earlier than the age of 40 and with the addition of a screening modality. In order to engage individuals at high risk on a punctual screening schedule, the ACR recommends that all women be evaluated for their risk of breast cancer by age 30. In this article, we discuss specific elements that would increase individuals' risk of breast cancer, models/calculators used to assess the individuals' risk for cancer subjectively, and the recommended screening schedule for those deemed at high risk.
Factors that contribute to the high risk of breast cancer
Specific germline genetic mutations are proven to increase an individual's risk of breast cancer. The most common and significant mutations implicated in breast cancer are BRCA1 and BRCA2, noticed with greater incidence in the Ashkenazi Jewish population. Other notable genetic mutations include TP53, CHEK2, PTEN, CDH 1, STK 11, PALB2, and ATM mutations. Despite recent advances and the adoption of genetic sequencing technology, offering genetic testing to the general population is resource-prohibitive. To this end, individuals with a family history of early-onset breast cancer should consider genetic testing upon discussion with their primary care provider. Of note, documented genetic mutation of the above listed is not essential to be considered high risk - the number of family members with a history of breast cancer, especially in first-degree relatives, places individuals at higher risk even without an established genetic mutation. Additional elements increase risk in individuals in addition to family history. Individuals previously exposed to chest radiation; specifically those who have received greater than 10 Gray of radiation and before the age of 30, are at increased risk of developing breast cancer. The risk of breast cancer notably increases approximately eight years after radiation exposure. Individuals with a history of prior breast cancer are at increased risk of developing recurrent or new breast cancer. Individuals with a history of high-risk lesions are also at increased risk of developing breast cancer. Individuals with category C or D breast densities, which denote heterogeneously dense or extremely dense breasts, are at an increased risk of developing breast cancer compared to non-dense counterparts.
Statistical models for risk assessment
The most commonly used models for risk assessment include but are not limited to the modified Gail, Claus, BRCAPRO, and Tyler-Cuzick or IBIS models. Each model weighs risk factors differently with proven validation in different patient populations. Hence, these models confer distinct advantages and shortcomings. Of importance, none of these models account for breast density as a contributing factor except the Tyler-Cuzick and Breast Cancer Surveillance Consortium model. The modified Gail model takes the patient's age, race, hormonal or reproductive history of the patient (for example, age at menarche and age at first live birth), history of breast disease, and family history into consideration, with validation in both the African-American and Caucasian ethnic groups. This model's major shortcoming is that it does not consider age at diagnosis of the first-degree relatives. The Claus model incorporates the number of relatives with breast cancer, the duration of diagnosis, and paternal family history. The shortcoming of this model is validation only in a Caucasian ethnic group. BRCAPRO calculates the probability that an individual is carrying BRCA1, BRCA2, or both mutations based on the individual's personal history of breast cancer or history of breast or ovarian cancer in first or second-degree relatives. The shortcoming of this model is not accounting for nonhereditary risk factors. The Tyler-Cuzick model considers factors such as family history, hormonal or reproductive history, and benign cancer disease and is considered the most accurate for predicting risk among all the models by ACR.
Recommended Screening Schedule
Individuals considered high risk per ACR, including individuals with a lifetime risk of 20% or more and genetic mutation carriers under untested breast relatives, should start annual screening mammogram at age 30. Currently, there are no age-based recommended upper limits for stopping screening. Individuals with a history of chest radiation before age 30 should start screening mammogram at 25 or 8 years after chest radiation, whichever is later. Similarly, there is no age-based upper limit for stopping screening. In those with a personal history of breast cancer before the age of 50 and dense breasts or a personal history of atypia or LCIS, annual screening mammogram starting at age of diagnosis is recommended.
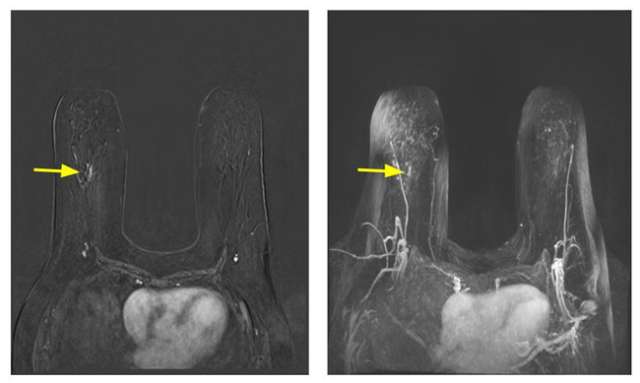
Contrast-enhanced breast MRI is more sensitive than mammography or ultrasound in high-risk populations. To this end, those considered to be high-risk, namely individuals with genetic mutations and their untested first-degree relatives, history of prior chest radiation, and calculated lifetime risk of 20% or more, are recommended to undergo contrast-enhanced breast MRI examination annually starting at the age of 25-30 years in addition to annual mammography. Supplemental ultrasound should replace MRI examination only in those who cannot tolerate MRI. Individuals with a history of breast cancer before age 50 or a personal history of breast cancer and dense breasts should also undergo an annual MRI examination and annual screening mammography. MRI examination can also be considered in individuals with a personal history of atypia or LCIS if additional risk factors exist. No specific recommendation for MRI screening exists for those individuals with dense breast tissue at this time. Annual MRI in high-risk populations is a complementary examination to mammography and should not replace it. Hence, screening MRI and mammography can be done concurrently or staggered by six months.
References
- Lee CH, Dershaw DD, Kopans D, Evans P, Monsees B, Monticciolo D, Brenner RJ, Bassett L, Berg W, Feig S, Hendrick E, Mendelson E, D'Orsi C, Sickles E, Burhenne LW. "Breast Cancer Screening with Imaging: Recommendations from the Society of Breast Imaging and the ACR on the Use of Mammography, Breast MRI, Breast Ultrasound, and Other Technologies for the Detection of Clinically Occult Breast Cancer." J Am Coll Radiol. 2010 Jan;7(1):18-27. DOI: 10.1016/j.jacr.2009.09.022. PMID: 20129267
- Lee CS, Monticciolo DL, Moy L. "Screening Guidelines Update for Average-Risk and High-Risk Women." AJR Am J Roentgenol. 2020 Feb;214(2):316-323. DOI: 10.2214/AJR.19.22205. Epub 2019 Nov 12. PMID: 31714845
- Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD, D'Orsi C, Harvey JA, Hayes MK, Huynh PT, Jokich PM, Lee SJ, Lehman CD, Mankoff DA, Nepute JA, Patel SB, Reynolds HE, Sutherland ML, Haffty BG. "ACR Appropriateness Criteria Breast Cancer Screening." J Am Coll Radiol. 2016 Nov;13(11S):R45-R49. DOI: 10.1016/j.jacr.2016.09.021. PMID: 27814813
- Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. "Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR." J Am Coll Radiol. 2018 Mar;15(3 Pt A):408-414. DOI: 10.1016/j.jacr.2017.11.034. Epub 2018 Jan 19. PMID: 29371086