Institute for Urologic Oncology
Artificial intelligence tool helps predict who will benefit from focal therapy for prostate cancer
Doctors now have a new method to determine the volume of cancer within a prostate tumor. A study led by UCLA investigators, Dr. Wayne Brisbane and Dr. Leonard Marks, shows that artificial intelligence (AI) could play a key role in improving treatment outcomes for men with prostate cancer by helping physicians determine who is most likely to benefit from partial gland cryoablation, a minimally invasive procedure that treats localized prostate tumors.

Shorter radiation therapy after prostate surgery safe, study finds
A new study published in JAMA Oncology and led by UCLA Health Jonsson Comprehensive Cancer Center investigators, Dr. Amar Kishan and Dr. Michael Steinberg, suggests there may be a faster option than previously used radiation methods. Researchers found that stereotactic body radiotherapy (SBRT), a form of high-dose radiation delivered in just five sessions, is as safe as conventional treatment, with similar side effects and a similar impact on quality of life.
Study uncovers genetic drivers of aggressive prostate cancer
Dr. Paul Boutros, professor of urology and human genetics at the David Geffen School of Medicine at UCLA, director of cancer data science at the UCLA Health Jonsson Comprehensive Cancer Center, is the co-senior author of a new study that helped identify 223 mutations that determine how prostate tumors develop and become lethal.
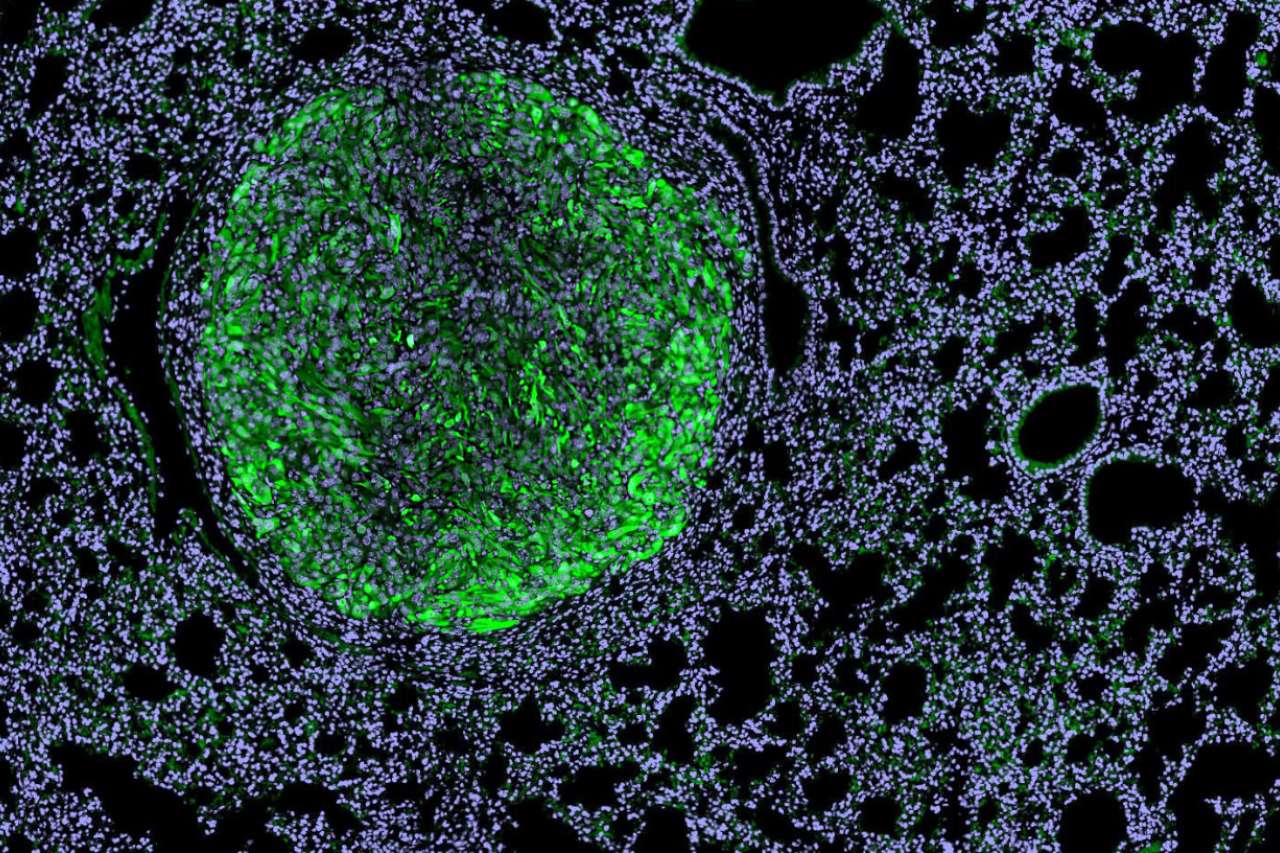
UCLA scientists develop off-the-shelf immunotherapy for metastatic kidney cancer
Dr. Arnold Chin, professor of urology, and Dr. Lily Wu, professor of molecular and medical pharmacology and urology at the David Geffen School of Medicine at UCLA, are co-senior authors on a new study that offers a promising next-generation strategy for treating metastatic renal cell carcinoma. UCLA researchers have developed a new kind of immunotherapy that uses specially engineered immune cells equipped with built-in weapons to attack kidney cancer tumors and reprogram their protective environment — all without the need to customize treatment for each individual patient.
Clinical Trials Spotlight
UCLA Health has a phase II trial to test if people who receive intismeran autogene and pembrolizumab are alive and cancer free longer than those who receive placebo and pembrolizumab, and to learn about the safety of intismeran autogene, pembrolizumab, and EV, and if people tolerate them. Karim Chamie, MD, Associate Professor of Urology, is the principal investigator.

