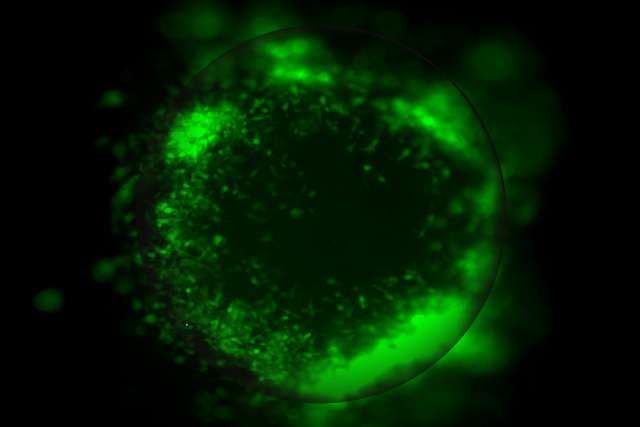
UCLA scientists have developed advanced miniature 3D tumor organoid models that make it possible to study glioblastoma tumors in a setting that closely mirrors the human brain, shedding light on how the aggressive cancer interacts with surrounding brain cells and the immune system to become more invasive and resistant to therapy.
The organoid models, described in two complementary studies published in Cell Reports, are built from human stem cells and recreate the complex mix of cell types found in the human brain. This approach allows researchers to directly observe how patient-derived tumors communicate with healthy brain tissue, revealing vulnerabilities that could be targeted with more personalized therapies.
“Glioblastoma has been incredibly difficult to treat in part because we haven’t had good ways to study how tumors behave in a truly human brain environment,” said senior author of the studies Aparna Bhaduri, PhD, assistant professor of medicine and biological chemistry at the David Geffen School of Medicine at UCLA and investigator at the UCLA Health Jonsson Comprehensive Cancer Center. “These new models developed by my lab help us understand how interactions with brain tissue and immune cells contribute to therapy failure, which is crucial for developing more effective, patient-specific treatments.”
Model helps identify hidden regulator of tumor aggressiveness
The first model, called the Human Organoid Tumor Transplantation (HOTT) system, reveals how glioblastoma communicates with surrounding brain cells to change its identity, invade tissue and resist treatment.
Using the HOTT system, researchers were able to closely observe how tumors interact with their microenvironment, uncovering a key communication pathway between tumor cells and surrounding brain cells. They found PTPRZ1, a protein expressed by both tumor cells and nearby brain cells in glioblastoma tissue, as a major regulator of tumor behavior that helps determine how aggressive the tumor becomes.
When the researchers reduced PTPRZ1 levels specifically in the brain cells of the organoids, they observed significant changes in the tumor cells, even though the tumor cells themselves were not directly altered. The tumor cells shifted into a more aggressive, invasive state, activating genes linked to movement and tissue invasion. They also formed longer tumor microtubes, thin cellular extensions that help tumors spread through the brain and resist treatment. These effects, surprisingly, did not depend on PTPRZ1’s usual enzyme activity, revealing a previously unrecognized role for the protein as a signaling mediator within the tumor environment.
“This study shows that glioblastoma is strongly influenced by the surrounding brain cells, not just the cancer itself,” said Bhaduri, who is also a member of the UCLA Broad Stem Cell Research Center. “By identifying PTPRZ1 as a new regulator of tumor behavior, we’re revealing hidden communication pathways and demonstrating how these organoid models can help uncover more effective therapeutic targets.”
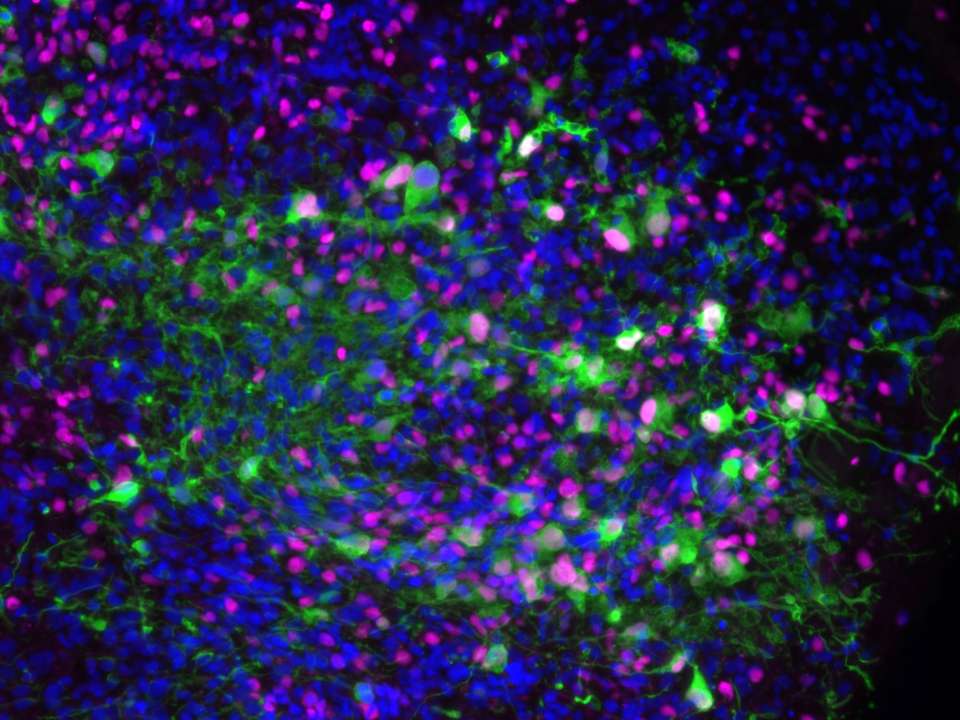
Tumor-immune model mimics how glioblastoma responds to immunotherapy
The second model, called the immune-human organoid tumor transplantation (iHOTT) model, builds on the HOTT system by incorporating components of the immune system into the organoids, enabling scientists to study how immune cells influence tumor growth and therapy resistance in a way that realistically mimics how glioblastoma responds to immunotherapy.
This approach preserves key features of both the tumor and immune cells, including CD4 and CD8 T cells, B cells, NK cells, and myeloid cells, allowing researchers to see how the cells communicate, how immune cells behave, and which populations expand or shut down in response to the tumor.
To test the system, the researchers treated the organoids with pembrolizumab, a PD-1 checkpoint inhibitor commonly used in cancer immunotherapy. They found that the drug activated the immune system, boosting CD4 T cells, B cells and immune signaling, yet tumor cells continued to survive and grow. So, while pembrolizumab can “wake up” the immune system, that alone is not enough to destroy glioblastoma.
“These immune changes observed in the lab closely mirrored what happens in real patients treated with pembrolizumab,” said Bhaduri. “The same shifts in immune cell populations occurred, the same communication pathways between immune cells were activated, and even rare or unconventional immune cell types expanded in similar ways. This demonstrates that iHOTT faithfully reproduces patient-like immune responses in a human-relevant system.”
By sequencing T cell receptors, the team also found that pembrolizumab increased the diversity of T cells, but the new T cells were unique to each individual rather than shared across patients. The strongest expansion occurred in CD4 T cells with a “stem-like” profile capable of generating continuous immune responses. These findings help explain why PD-1 inhibitors show limited benefit in glioblastoma, as each patient’s immune system reacts differently and very few T cells recognize shared tumor targets.
These findings, the researchers noted, show how organoid models can uncover mechanisms that are otherwise hidden in standard animal models and cell cultures.
“Together, these studies show that these patient-specific organoid models offer a powerful tool to uncover hidden tumor interactions and test new therapies, bringing personalized treatment for this deadly cancer a step closer,” said Bhaduri.
***
The first authors of the HOTT model study are Weihong Ge and Ryan Kan from the Bhaduri laboratory. Other UCLA authors are Can Yilgor, Elisa Fazzari, Patricia Nano, Daria Azizad, Heer Shinglot, Matthew Li, Joyce Ito, Christopher Tse, Hong Tum, Jessica Scholes, Shivani Baisiwala, Kunal Patel and David Nathanson.
The first author of the iHOTT model study is Shivani Baisiwala, a neurosurgery resident at UCLA. Other UCLA authors are Elisa Fazzari, Matthew Li, Antoni Martija, Daria Azizad, Lu Sun, Gilbert Herrera, Trinh Phan, Amber Monteleone, Ryan Kan, David Nathanson, Anthony Wang, Won Kim, Richard Everson, Kunal Patel, Linda Liau and Robert Prins.
The work for both the studies were funded in part by the National Cancer Institute, Swim Across America, the UCLA Health Jonsson Comprehensive Cancer Center, a Sloan Research Fellowship, the Sontag Foundation, a V Scholar Award, the Uncle Kory Foundation, the American Cancer Society, the Margaret Early Medical Research Trust, the Pew Foundation, the Alexander and Margaret Stewart Trust, a McKnight Neurobiology of Brain Disorders Award, the Rose Hills Foundation and the UCLA Broad Stem Cell Research Center.