Ultrasound: Associated Features
by Katie McNulty, MD, and Jane Dascalos, MD
Introduction
Certain associated features of lesions seen sonographically play an important role in the final assessment (BIRADS categorization) of the lesion. These features include architectural distortion, duct changes, skin changes, edema, vascularity, and elasticity.
Architectural Distortion
The definition of architectural distortion as per the 5th Edition of the BIRADS Atlas is when “the normal architecture of the breast is distorted with no mass visible.” Architectural distortion is most often noted on screening mammography and subsequently further evaluated via diagnostic mammography and ultrasound. There are many causes of distortion, both benign and malignant, but it may be the first presentation of a developing malignancy and is often a subtle finding which may be missed by inexperienced interpreters.

Architectural distortion is a focal alteration in the normal pattern of breast parenchyma. A more relatable example is of the plant with the radiating bands of sharp leaves surrounded by normal shrubbery above. Think of the normal shrubbery as the normal breast tissue, and the area of distortion as the plant in the center. While there may be no obvious mass in the breast tissue, there is clearly a difference between the normal and distorted tissue. The plant in the center has its leaves radiating from a single point, which, in the breast, may represent a developing mass which is “tugging” on the normal tissue. Let’s compare this analogy to a real-life example on sonography:
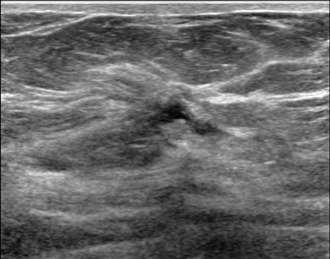
The ultrasound image above demonstrates sonographic architectural distortion. The hyperechoic (bright) bands of tissue interdigitating between the fat in the breast become focally altered and seem to radiate around a small lesion. Biopsy revealed a complex sclerosing lesion (sometimes called a radial scar) which is a benign, but sometimes high risk lesion, which can be recognized as architectural distortion on ultrasound. Other examples of benign causes of architectural distortion include sclerosing adenosis, fat necrosis, post-procedural changes, granular cell tumors, and breast fibromatosis. Architectural distortion may also be associated with breast cancer, as evidenced by the example below:
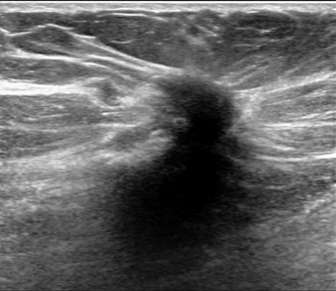
The above ultrasound demonstrates a hypoechoic (dark) mass with indistinct margins, posterior shadowing, and distortion of the normal breast tissue. This was biopsied, revealing invasive ductal carcinoma (IDC). Both ductal carcinoma in situ (DCIS) and IDC may result in distortion.
Duct Changes
Normal milk ducts, which function to carry the milk produced by the lobular (glandular) tissue to the nipple, are anechoic (create no ultrasound echoes and are black), with regular margins, and parallel in course to other mammary structures on sonography. Changes in the ducts, especially those which are focal or asymmetric, are important sonographically because they may suggest underlying pathology. Benign causes of duct changes include hormonal changes (pregnancy/lactation) and benign duct ectasia (also known as periductal or plasma cell mastitis).
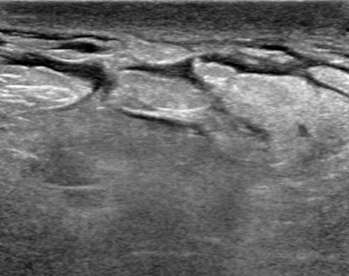
The ultrasound above demonstrates a well-circumscribed, anechoic, parallel, and avascular tubular structure with well-defined borders, consistent with a dilated duct. The opposite breast also revealed similar dilation of the ducts, this symmetry is reassuring and benign. Features of ductal dilation which are concerning (and may warrant biopsy) include unilateral breast ductal dilation or a focal ductal dilation in a breast with otherwise normal sized ducts. Furthermore, a dilated duct which is not anechoic (fluid filled or with internal soft tissue nodularity), with an irregular shape, or with indistinct margins are also more suspicious.
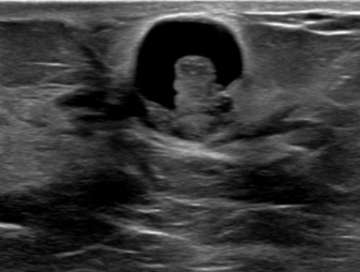
The ultrasound above demonstrates a well-circumscribed, anechoic, parallel, and avascular tubular structure with well-defined borders, consistent with a dilated duct. The opposite breast also revealed similar dilation of the ducts, this symmetry is reassuring and benign. Features of ductal dilation which are concerning (and may warrant biopsy) include unilateral breast ductal dilation or a focal ductal dilation in a breast with otherwise normal sized ducts. Furthermore, a dilated duct which is not anechoic (fluid filled or with internal soft tissue nodularity), with an irregular shape, or with indistinct margins are also more suspicious.
Skin Changes
Thickening: Normal breast skin measures under 2.5 mm in thickness; any increase in skin thickness should be noted and taken into consideration when formulating the BIRADS categorization. Benign causes of skin thickening may include skin lesions such as a dermoid (sebaceous) cyst or changes from prior radiation therapy. However, skin thickening may be related to infection (cellulitis/mastitis or abscess) or underlying malignancy. Below is an example of a breast abscess with marked thickening of the overlying skin (especially compared to the normal-thickness skin on the left side of the image.
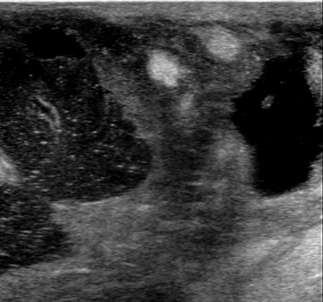
Retraction: Any focal invagination of the skin (often noted as nipple inversion or skin dimpling on physical exam) should be noted, because, like with architectural distortion, this may suggest deeper pathology. Below is an ultrasound demonstrating nipple retraction with an underlying heterogeneous mass with irregular contours. This lesion was highly suspicious (BIRADS 5) and proven to be invasive ductal carcinoma on biopsy.
Edema
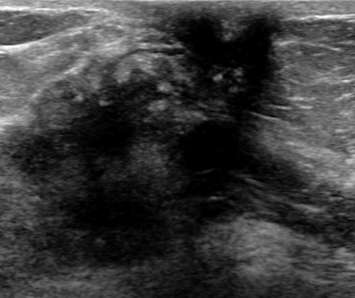
Tissue swelling (edema) can be seen in systemic and local (benign or malignant) processes. Edema in the breast manifests as skin and trabecular thickening (enlargement of the areas of normal breast connective tissue due to increased extracellular fluid). Bilateral edema suggests a systemic condition such as congestive heart failure. Unilateral edema may be iatrogenic (i.e. post-surgical or radiation changes), infection (i.e. abscess), or tumoral venous or lymphatic obstruction (i.e. inflammatory breast cancer). Below is an example of skin and trabecular thickening, with increased fluid (hypoechoic) surrounding normal fat lobules within the breast. Notice that there is decreased penetration (deep haziness) on this exam due to the edema (increased density of the breast tissue); this is an incidental finding in a patient with congestive heart failure.
Vascularity
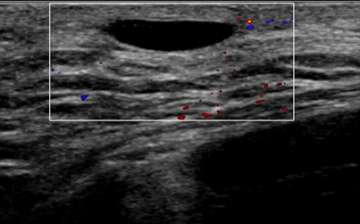
Using Doppler ultrasound, the amount of blood flow to an area of interest can be assessed and compared to surrounding tissue. Increased vascularity may suggest inflammation (i.e. infection such as mastitis) or malignancy. Doppler is especially helpful when evaluating for blood flow to cystic lesions, which may suggest a solid component (vascular) rather than debris within a cyst (avascular). In Figure 4, the anechoic structure was avascular on Doppler, consistent with a cystic structure (dilated duct).
Elasticity
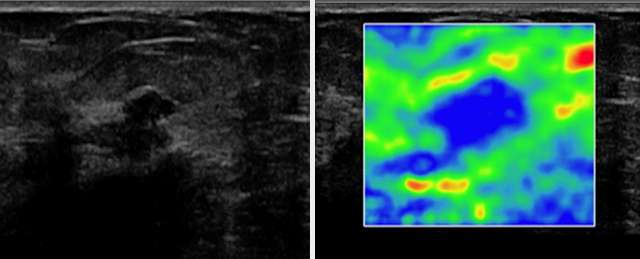
Another useful tool in conjunction with Doppler sonography is ultrasound elastography (sono-elastography), which uses sound waves to assess the mechanical properties of tissues (firmness). Soft lesions “deform” from sound waves more so than firm lesions. In general, malignant lesions tend to be firmer (deform less) than benign lesions. One application of this property is with Strain Elastography, where a color scale can be used to give a qualitative analysis of the firmness of a lesion. The two figures below, courtesy of the article “The Role of Sonoelastography in Breast Lesions” (Richard Barr, et. al), give an example of Strain elastography in evaluating a hypoechoic breast mass with irregular borders in a 63-year-old woman (left image). On elastography (right image), blue represents stiffer breast tissue and red, softer tissue. This lesion is blue, indicating a firm lesion, correctly increasing suspicion, as this lesion was biopsied and proven to be invasive ductal carcinoma.
Citations
- D’Orsi CJ, Sickles EA, Mendelson EB, Morris EA, et al. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology; 2013
- Gaur, Shantanu, et al. “Architectural Distortion of the Breast.” American Journal of Roentgenology, vol. 201, no. 5, 2013, DOI: 10.2214/AJR.12.10153
- Dogan, B.E., Ceyhan, K., Tukel, S., Saylisoy, S. and Whitman, G.J. (2005), “Ductal Dilatation as the Manifesting Sign of Invasive Ductal Carcinoma.” Journal of Ultrasound in Medicine, 24: 1413-1417. DOI: 10.7863/jum.2005.24.10.1413
- Catherine S. Giess, Sughra Raza, and Robyn L. Birdwell. “Distinguishing Breast Skin Lesions from Superficial Breast Parenchymal Lesions: Diagnostic Criteria, Imaging Characteristics, and Pitfalls.” RadioGraphics 2011 31:7, 1959-1972. DOI: 10.1148/rg.317115116
- Kwak, J. Y., Kim, E. K., Chung, S. Y., You, J. K., Oh, K. K., Lee, Y. H., Kwon, T. H., & Jung, H. K. (2005). “Unilateral breast edema: spectrum of etiologies and imaging appearances.” Yonsei medical journal, 46(1), 1–7. DOI: 10.3349/ymj.2005.46.1.1
- Ramos, R.M. Lorente, et al. Educational Exhibit: “Breast Edema. A Pictorial Review with Pathologic Correlation.” European Society of Radiology, DOI: 10.1594/ecr2014/C-0911
- Imtiaz S. “Breast elastography: A new paradigm in diagnostic breast imaging.” Appl Radiol. 2018;47(3):14-19. DOI: 10.1088/0031-9155/48/4/304
- Richard G. Barr and Zheng Zhang. “Shear-Wave Elastography of the Breast: Value of a Quality Measure and Comparison with Strain Elastography”. Radiology 2015 275:1, 45-53. DOI: 10.1148/radiol.14132404
- Barr, Richard G. “The Role of Sonoelastography in Breast Lesions.” Seminars in Ultrasound, CT and MRI, vol. 39, no. 1, 2018, pp. 98–105., DOI: 10.1053/j.sult.2017.05.010.