Case: Atypical Ductal Hyperplasia
By: Zachary Winchester MD, and Nooshin Najmi MD
Introduction and Epidemiology:
Atypical ductal hyperplasia (ADH) is a non-malignant but high-risk lesion associated with progression to more advanced neoplasms including ductal carcinoma in situ (DCIS) and invasive carcinoma, and as a marker for the development of additional breast cancer. Approximately 3-5% of female breast biopsies will contain ADH, with most lesions found in women in their 40s due to the onset of screening mammograms. Although quite rare, ADH can also be seen in the male breast, with prevalence of ADH in males with gynecomastia cited to be less than 1%.
Genetics and Risk Factors:
Estrogen exposure is the primary risk factor for the development of atypical ductal hyperplasia. Estrogen and its metabolites have associated DNA-damaging effects, which lead to abnormal growth control and unchecked proliferation within breast tissue. Although many genes have been linked to breast oncogenesis, the sequence of genomic changes in ADH are not precisely known at this time. However, accumulated genomic changes throughout a patient’s lifetime via exposure to estrogen are likely the major player behind the development of ADH and other pre-malignant and malignant lesions. Molecular studies have shown that similar genomic changes are found in both ADH and low-grade DCIS, leading to the hypothesis that ADH and DCIS lie on a spectrum of disease and that ADH represents the early pattern of disease or the precursor lesion to DCIS. Additional risk factors for ADH are similar to those for invasive carcinoma, including a family history of breast cancer and BRCA1/BRCA2 mutations.
Clinical Presentation:
Atypical ductal hyperplasia is usually found incidentally on routine mammograms or breast MRI screenings. Although symptomatology and physical examination are vital to diagnosing breast pathology in general, ADH is usually too small to be appreciated upon palpation or to cause symptoms.
Pathology:
The pathologic definition of ADH is that of a single clonal intraductal epithelial cell proliferation that partially or fills ≤2 membrane-bound ductal spaces, or occupies ≤2 mm in maximum dimension. Although histopathologic features are similar to low-grade DCIS, it is purely the size and/or number of ductal spaces involved that differentiates the two. If the findings involve a size of >2 mm or >2 separate ductal spaces, low-grade DCIS is the more appropriate diagnosis. Histologic examination shows well-defined, monomorphic cells with small, rounded, and evenly spaced nuclei with rare mitoses. Large nuclei with frequent mitoses, such as those in intermediate- or high-grade DCIS, are not seen with ADH. Architectural features are similar to low-grade DCIS, including arcades and rigid bridges of uniform thickness, and solid, cribriform, and micropapillary growth patterns. Immunohistochemistry demonstrates strong and uniform estrogen receptor expression.
Radiographic Features:
The mammographic appearance of ADH is usually that of grouped or regional calcifications, most often of the amorphous or coarse heterogeneous subtype. ADH can also be less commonly present as a mass or focal asymmetry on screening mammograms. As calcifications are the most common presentation of ADH on screening mammograms, and as ultrasound suboptimally evaluates areas of calcification, ADH is less often seen on ultrasound examinations. However, the most common sonographic feature of lesions demonstrating ADH on ultrasound-guided biopsies includes a hypoechoic, microlobulated mass without acoustic transmission or shadowing. The most common appearance of ADH on breast MRI is nonmass enhancement in a focal, linear, segmental, or regional distribution. The second-most common appearance is that of an enhancing mass.
Treatment and Prognosis:
Studies suggest surgical upgrade rates to DCIS or invasive carcinoma of up to 22-65% for biopsy-proven ADH. Thus, surgical excision is recommended for cases of ADH found on core needle biopsy. Ongoing research is being performed to identify which subtypes of lesions are associated with surgical upgrades. Thus, in the future, we might be able to stratify patients with biopsy-proven ADH into those who require surgical excision, or those who can be monitored over time without the need for excisional biopsy. However, such research is limited in scope at this time, and surgical excision for all cases of ADH on core needle biopsy is currently recommended. The prognosis usually depends on the final excisional biopsy results.
Cases:
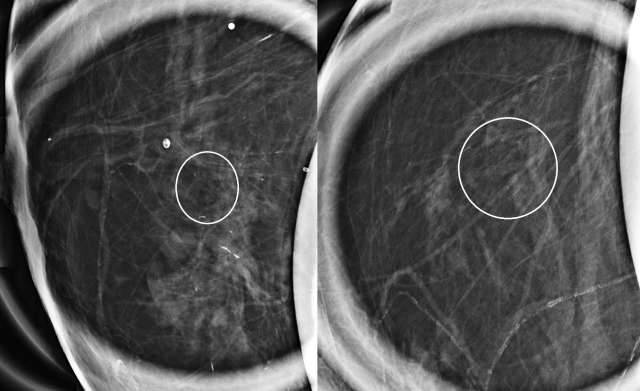
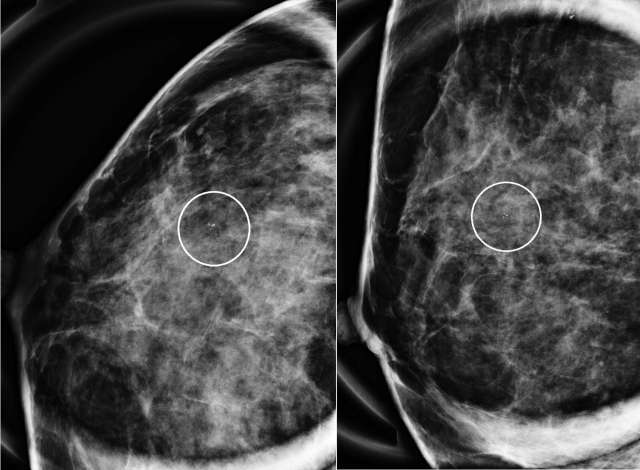

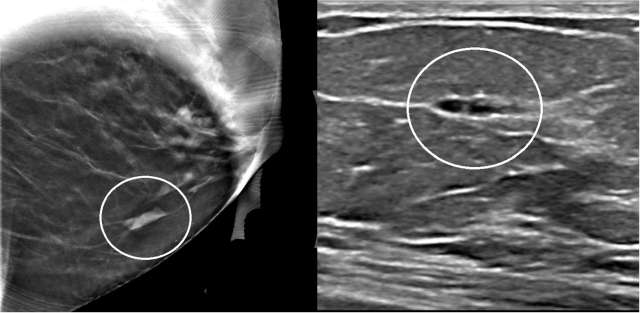
References
- Tomlinson-Hansen S, Khan M, Cassaro S. Atypical Ductal Hyperplasia. 2023 Apr 24. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan–. PMID: 32965915. Bookshelf ID: NBK562244
- Myers DJ, Walls AL. Atypical Breast Hyperplasia. 2023 Feb 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. PMID: 29261937. Bookshelf ID: NBK470258
- Page DL, Rogers LW. "Combined Histologic and Cytologic Criteria for the Diagnosis of Mammary Atypical Ductal Hyperplasia." Hum Pathol. 1992 Oct;23(10):1095-7. DOI: 10.1016/0046-8177(92)90026-y. PMID: 1328030
- Lapid O, Jolink F, Meijer SL. "Pathological Findings in Gynecomastia: Analysis of 5113 Breasts." Ann Plast Surg. 2015 Feb;74(2):163-6. DOI: 10.1097/SAP.0b013e3182920aed. PMID: 23788148
- Lewin AA, Mercado CL. "Atypical Ductal Hyperplasia and Lobular Neoplasia: Update and Easing of Guidelines." AJR Am J Roentgenol. 2020 Feb;214(2):265-275. DOI: 10.2214/AJR.19.21991. Epub 2019 Dec 11. PMID: 31825261