Case: Invasive Lobular Carcinoma
by: Julia Gerras, MD, Nooshin Najmi, MD.
Epidemiology:
Invasive lobular carcinoma (ILC) is the second most common invasive breast cancer accounting for 5-15% of invasive breast cancers behind invasive ductal carcinoma (IDC). ILC is typically seen in post-menopausal women, peaking at age 50-601. It is more common in white individuals but has a higher mortality rate in African American individuals as they have a higher predisposition to have grade 3 ILC. Additionally, with the increased rate of post-menopausal hormone therapy, the incidence of ILC has increased over the last 20 years2.
Genetics and Risk Factors:
As with other cancers, genetics play a role in predisposition to developing ILC. Specifically, mutations in CDH1, which codes for E-cadherin, is directly linked to ILC. This will be elaborated upon later in this discussion. BRCA2 mutations are linked with both lobular and ductal carcinoma, while BRCA1 and TP53 mutations are mostly related to ductal carcinomas. In addition to genetic mutations, there are other risk factors to developing ILC. As alluded to above, ILC is related to hormonal exposure, thus making it more variable in incidence. It is associated with increased estrogen exposure such as early menarche, late menopause, and late age at first birth2. Alcohol consumption and higher BMI in post-menopausal women is associated with increased risk of breast cancer in general, though not specific to ILC.
Clinical Presentation:
The clinical features of ILC are far more varying and less defined versus its counter-part IDC. Patients with ILC may present with a vague palpable area of breast tissue that appears more thickened or swollen compared to the remaining breast tissue, nipple changes, skin dimpling, or asymmetric breast size1. Given its vague clinical and radiographic features, ILC is more difficult to detect and ultimately manage, resulting in 67% of these patients presenting with metastatic disease1. Compared to IDC, ILC is far more likely to metastasize to the peritoneum or retroperitoneum, gastro-intestinal tract, urogenital tract, leptomeninges, and myocardium. The metastatic rate to the liver, bone, and pleura is comparable to that of IDC3. Additionally, ILC has a greater rate of involving the contralateral breast resulting in bilateral cancer when compared to IDC; the bilateral involvement is reported at 20-29%4.
Pathology:
Under the microscope, ILC consists of small, round, and nucleated cells that invade circumferentially around both the breast lobules and ducts in a single-file manner. About 80% of cases lack E-cadherin, which plays a vital role in creating the adhesive layer between epithelial cells that generally gives tumor growth their uniform, cohesive shape5. Without E-cadherin, ILC tends to grow more diffusely and often without a significant desmoplastic reaction. This accounts for the difficulty in its detection on physical examination, imaging modalities, and even pathologic evaluation6. However, ILC is often estrogen receptor positive, progesterone receptor positive, and human epidermal growth factor 2 (HER2) negative, making it favorable for hormone therapy. Lastly, there are multiple variants of ILC, the most common of which is the classic variant that is typically histological grade 2. The other special morphological subtypes include pleomorphic, solid, alveolar, and tubulo-lobular7.
Radiographic features:
- Mammography/Digital Breast Tomosynthesis (DBT):
ILC cells preserve the architecture of ducts, limiting the detection of mammography. The sensitivity of mammography is 57-81%, with up to 30% of cases not at all visible, while the specificity is 34%1. The most common mammographic presentations are a mass with spiculated or indistinct margins (44%-65%) and architectural distortions (10-34%)1. Some less common findings are asymmetries, focal asymmetries, and shrinking breast appearance over time. DBT produces thinner slices and thus reduces the tissue masking effect of mammography. These two modalities are described together in the literature for ILC detection rates.
- Ultrasound (US):
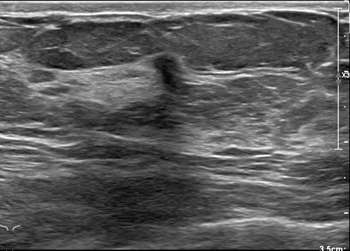
Ultrasound has a higher sensitivity for detecting ILC than mammography, reported at 68-98%. A hypoechoic irregular mass with posterior shadowing is the most common imaging finding on US (60%)1. Less common findings are irregular shape and angular margins, or posterior shadowing without a discrete mass. About 10% of the time, ILC is not detected on ultrasound1.
- MRI:
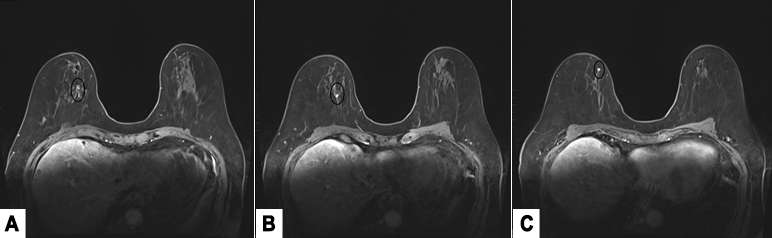
MRI is the most sensitive modality to detect ILC especially for detecting bilateral cancers, with a sensitivity of 93-95%. The most common presentation is a solitary mass with spiculated or irregular margins (95%). Non-mass enhancement is less common (69%)1. On Kinetic imaging, there will be delayed enhancement and less washout in the delayed phase when compared to that of IDC. If a patient has biopsy proven ILC, often the next step is to get an MRI to examine the contralateral breast as ILC is often multifocal and multicentric.
- Contrast Enhanced Spectral Mammography:
Contrast Enhanced Spectral Mammography (CESM) can be used to detect ILC. CESM detects breast-enhancing lesions like that of MRI, though is less time consuming for both the patient and the radiologist reading the study; it is also cheaper8. The overall sensitivity of CESM is 97%, with a higher sensitivity than MRI to detect multifocal, multicentric disease9. Thus, CESM is a good alternative to MRI in patients with claustrophobia or implants that are not compatible with MRI.
- 18F-Fluoroestradiol PET/CT:
18F-Fluoroestradiol (FES) is a radiotracer used in nuclear medicine that has uptake in tissues expressing estrogen receptor (ER). Since ILC is ER positive 95% of the time, this study can be useful in detecting primary lesions, as well as staging disease10. This is an emerging modality, as FDG PET/CT has more historically been used in staging exams with a 60% detection rate. FES PET/CT has shown 88% detection rate of known ILC lesions11.Similarly, FES PET/CT detected 71% more metastatic lesions when compared to FDG PET/CT10.
Treatment and Prognosis:
Surgical excision with clear margins, often-times a mastectomy, is pursued in patients with ILC due to the propensity for this type of cancer to be multicentric within the breast. In patients with early-stage cancer, breast-conserving surgery in combination with post-operative radiation is an alternative. Other treatment options in early stage ILC is targeted hormone therapy since ILC is ER positive, and endocrine therapy such as aromatase inhibitors, specifically in post-menopausal women, to stop the conversion of androgens to estrogen. ILC has been shown to be less responsive to chemotherapy. In the setting of metastatic ILC, endocrine therapy has been shown to be the most crucial to management12.
Case 1:
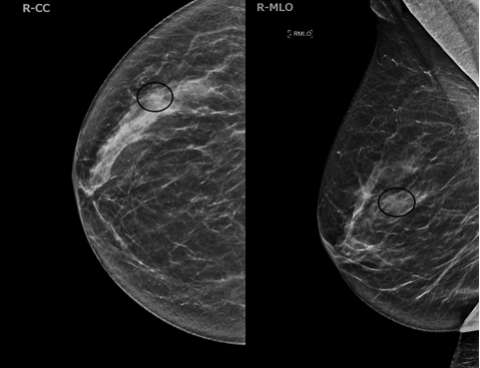
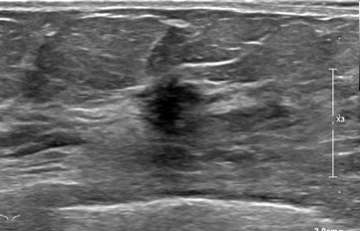
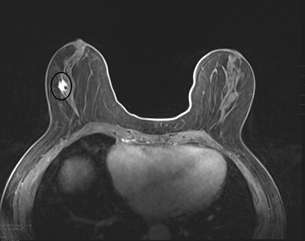
Case 2:
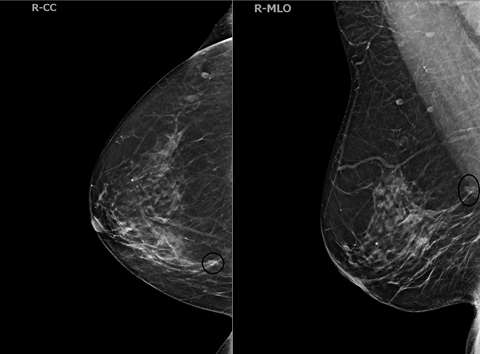
References
- Manning P, Fazeli S, Lim V, Ladd WA, Eghtedari M, Chong A, Rakow-Penner R, Ojeda-Fournier H. "Invasive Lobular Carcinoma: A Multimodality Imaging Primer." Radiographics. 2022 Jul-Aug;42(4):E115-E116. DOI: 10.1148/rg.210058.
- Dossus L, Benusiglio PR. "Lobular Breast Cancer: Incidence and Genetic and Non-genetic Risk Factors." Breast Cancer Res. 2015 Mar 13;17:37. DOI: 10.1186/s13058-015-0546-7.
- Mathew A, Rajagopal PS, Villgran V, Sandhu GS, Jankowitz RC, Jacob M, Rosenzweig M, Oesterreich S, Brufsky A. "Distinct Pattern of Metastases in Patients with Invasive Lobular Carcinoma of the Breast." Geburtshilfe Frauenheilkd. 2017 Jun;77(6):660-666. DOI: 10.1055/s-0043-109374. Epub 2017 Jun 28. PMID: 28757653
- Arpino G, Bardou VJ, Clark GM, Elledge RM. "Infiltrating Lobular Carcinoma of the Breast: Tumor Characteristics and Clinical Outcome." Breast Cancer Res. 2004;6(3):R149-56. DOI: 10.1186/bcr767. Epub 2004 Feb 17. PMID: 15084238
- Pramod N, Nigam A, Basree M, Mawalkar R, Mehra S, Shinde N, Tozbikian G, Williams N, Majumder S, Ramaswamy B. "Comprehensive Review of Molecular Mechanisms and Clinical Features of Invasive Lobular Cancer." Oncologist. 2021 Jun;26(6):e943-e953. DOI: 10.1002/onco.13734. Epub 2021 Mar 16. PMID: 33641217
- Johnson K, Sarma D, Hwang ES. "Lobular Breast Cancer Series: Imaging." Breast Cancer Res. 2015 Jul 11;17(1):94. DOI: 10.1186/s13058-015-0605-0. PMID: 26163296
- McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR. "Invasive Lobular Carcinoma of the Breast: The Increasing Importance of this Special Subtype." Breast Cancer Res. 2021 Jan 7;23(1):6. DOI: 10.1186/s13058-020-01384-6. PMID: 33413533
- Pereslucha AM, Wenger DM, Morris MF, Aydi ZB. "Invasive Lobular Carcinoma: A Review of Imaging Modalities with Special Focus on Pathology Concordance." Healthcare (Basel). 2023 Mar 3;11(5):746. DOI: 10.3390/healthcare11050746. PMID: 36900751
- Costantini M, Montella RA, Fadda MP, Tondolo V, Franceschini G, Bove S, Garganese G, Rinaldi PM. "Diagnostic Challenge of Invasive Lobular Carcinoma of the Breast: What Is the News? Breast Magnetic Resonance Imaging and Emerging Role of Contrast-Enhanced Spectral Mammography." J Pers Med. 2022 May 25;12(6):867. DOI: 10.3390/jpm12060867. PMID: 35743654
- Ulaner GA, Jhaveri K, Chandarlapaty S, Hatzoglou V, Riedl CC, Lewis JS, Mauguen A. "Head-to-Head Evaluation of 18F-FES and 18F-FDG PET/CT in Metastatic Invasive Lobular Breast Cancer." J Nucl Med. 2021 Mar;62(3):326-331. DOI: 10.2967/jnumed.120.247882. Epub 2020 Jul 17. PMID: 32680923
- Covington MF, Hoffman JM, Morton KA, Buckway B, Boucher KM, Rosenthal RE, Porretta JM, Brownson KE, Matsen CB, Vaklavas C, Ward JH, Wei M, Buys SS, Chittoria N, Yakish ED, Archibald ZG, Burrell LD, Butterfield RI, Yap JT. "Prospective Pilot Study of 18F-Fluoroestradiol PET/CT in Patients with Invasive Lobular Carcinomas." AJR Am J Roentgenol. 2023 Aug;221(2):228-239. DOI: 10.2214/AJR.22.28809. Epub 2023 Mar 15. PMID: 36919879
- Van Baelen K, Geukens T, Maetens M, Tjan-Heijnen V, Lord CJ, Linn S, Bidard FC, Richard F, Yang WW, Steele RE, Pettitt SJ, Van Ongeval C, De Schepper M, Isnaldi E, Nevelsteen I, Smeets A, Punie K, Voorwerk L, Wildiers H, Floris G, Vincent-Salomon A, Derksen PWB, Neven P, Senkus E, Sawyer E, Kok M, Desmedt C. "Current and Future Diagnostic and Treatment Strategies for Patients with Invasive Lobular Breast Cancer." Ann Oncol. 2022 Aug;33(8):769-785. DOI: 10.1016/j.annonc.2022.05.006. Epub 2022 May 21. PMID: 35605746