Case: Ductal Carcinoma In Situ
by Mark McArthur, MD and James Chalfant, MD
Introduction, Demographics, Clinical Presentation
Ductal carcinoma in situ (DCIS) is considered a noninvasive, precursor lesion to invasive breast cancer. The basic pathologic definition of DCIS is the presence of malignant epithelial cells within the mammary ducts and without invasion into or passed the basement membrane.1
The incidence of DCIS has increased with the advent of screening mammography. Prior to the establishment of screening mammography, DCIS accounted for only 5% of breast cancers; this number has risen to nearly 30% due to screening mammography.3 This speaks to the value of screening mammography as more cases are diagnosed during the precursor stage to invasive malignancy. It is important to note that some of these cases of DCIS will remain indolent, never progressing to IDC, but it is currently not possible to differentiate these subsets based on imaging.
There are several risk factors associated with DCIS. The most significant of these is age, with the vast majority of diagnoses occurring at age >50 years and peak incidence in the 65-69 age range. DCIS diagnosis is uncommon in the <30 age group. Other known risk factors include family history of breast cancer and nulliparity. There is either mixed data or lack of data establishing a relationship between DCIS diagnosis and hormone replacement therapy (HRT), contraceptive use, and smoking status. Incidence rates are similar between non-Hispanic white and non-Hispanic black populations and lower rates among Hispanic, American Indian, and Native Alaskan populations.4
Imaging Features
The imaging features of DCIS are nonspecific; however, there are several features which may be seen on mammography, ultrasound, and magnetic resonance imaging (MRI) which should result in suspicion for DCIS. The reason these features are suspicious is due to the anatomy of the terminal ductal lobule unit (TDLU). The main ducts of the mammary glands terminate in subsequently smaller branches, ultimately reaching a terminal duct leading to a lobule with multiple small acini.3
On mammography, the most suggestive feature of DCIS is calcification, which is seen in the majority of cases. The calcification morphologies frequently seen with DCIS are fine linear, fine pleomorphic, coarse heterogeneous, and amorphous.5 In particular, fine linear morphology directly corresponds to disease along a duct. The distribution of these calcifications also lends credence to a diagnosis of DCIS. Linear and segmental distribution patterns are associated with DCIS; these patterns reflect the spread of disease through the ducts and TDLUs (Figure 1).3 In fact, linear and segmental distributions may be related to malignancy up to 80% of the time.6
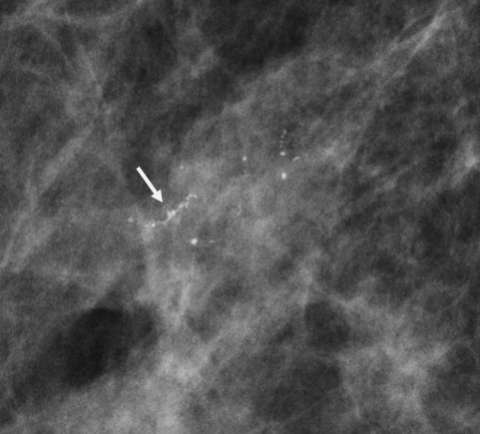
Calcifications are best visualized on mammography, and thus this modality plays a crucial role in screening for DCIS; however other imaging modalities may also provide additional information that can suggest a diagnosis of DCIS. Ultrasound may be utilized when calcifications are identified on mammogram to look for solid components, which may aid in ultrasound-guided biopsy. On ultrasound, calcified DCIS may appear as echogenic foci within a mass or duct. Noncalcified DCIS may have a similar appearance, but without posterior acoustic shadowing. In addition, internal vascularity can also help to improve the positive predictive value.7 On MRI, enhancement patterns play a large role, with linear or segmental non-mass enhancement most commonly associated with DCIS, regardless of MRI kinetics (Figure 2).8
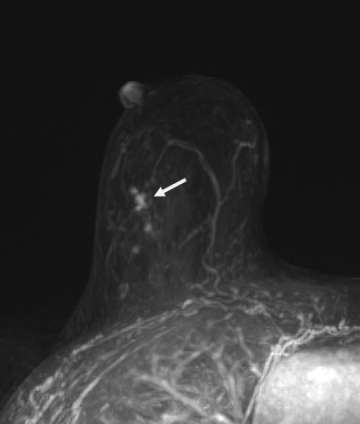
Pathology
On pathology, DCIS is categorized by architecture and nuclear grading. Due to the heterogeneity of DCIS lesions, there are a few recognized microscopic architectural patterns, including comedo, cribriform, micropapillary, solid, and mixed subtypes.9 By far, the most common pattern is a mixed subtype, which causes problems with reproducibility of findings.10 Despite this problem, there is value in identifying architectural subtypes from a clinical perspective. Micropapillary DCIS is more often multiquadrant compared to comedo-type patterns.11 In addition, cribriform architecture is most often first detected due to symptom onset, whereas comedo-type disease is commonly detected on mammogram.12
Due to the commonly mixed presentation of architectural subtypes of DCIS, newer systems of classification place an emphasis on nuclear grade. In general, DCIS is defined as monomorphic cells within the ductal lumen involving the TDLU that grow towards the nipple. The morphology of the nucleus within these cells defines their nuclear grade. Low-grade DCIS presents with small, regularly spaced cells containing round bland nuclei, no prominent nucleoli, and well-defined cell boundaries. High-grade DCIS contains large pleomorphic cells, poorly defined boundaries, and multiple nucleoli with regions of central necrosis. Intermediate-grade disease falls in between these two categories.9
Treatment
The mainstay of treatment for DCIS is surgical resection. Surgical resection (breast-conserving lumpectomy or mastectomy) may also be paired with sentinel lymph node biopsy if the patient is high risk, there is a large component of high-grade DCIS, or patient is undergoing mastectomy.
After surgery, radiation therapy targeted to the surgical bed is often performed.13 However, there is some evidence that low and intermediate grade DCIS can be watched with active surveillance rather than treated with radiation due to relatively low recurrence rates. With active surveillance, any new masses, asymmetries, or new grouped calcifications warrant further diagnostic imaging and biopsy. In addition, those tumors which are estrogen receptor positive are treated with tamoxifen (or aromatase inhibitors in postmenopausal women) in order to decrease the risk of recurrence. Ongoing research is being conducted to further enable tailored therapy for different patient and tumor characteristics.14
References
- Vaidya Y, Vaidya P, Vaidya T. "Ductal Carcinoma In Situ of the Breast." Indian J Surg. 2015 Apr;77(2):141-6. DOI: 10.1007/s12262-013-0987-0.
- Pinder SE, Ellis IO. "The Diagnosis and Management of Pre-invasive Breast Disease: Ductal Carcinoma in situ (DCIS) and Atypical Ductal Hyperplasia (ADH) – Current Definitions and Classification." Breast Cancer Research. 2003;5(5):254-7. DOI: 10.1186/bcr623.
- D’orsi CJ. "Imaging for the Diagnosis and Management of Ductal Carcinoma in situ." J Natl Cancer Inst Monogr. 2010;2010(41):214-7. DOI: 10.1093/jncimonographs/lgq037.
- Parikh U, Chhor CM, Mercado CL. "Ductal Carcinoma in situ: The Whole Truth." AJR Am J Roentgenol. 2018 Feb;210(2):246-255. DOI: 10.2214/AJR.17.18778.
- Dershaw DD, Abramson A, Kinne DW. "Ductal Carcinoma in situ: Mammographic Findings and Clinical Implications". Radiology. 1989 Feb;170(2):411-5. DOI: 10.1148/radiology.170.2.2536185.
- Liberman L, Abramson AF, Squires FB, Glassman JR, Morris EA, Dershaw DD. "The Breast Imaging Reporting and Data System: Positive Predictive Value of Mammographic Features and Final Assessment Categories." AJR Am J Roentgenol. 1998 Jul;171(1):35-40. DOI: 10.2214/ajr.171.1.9648759.
- Wang LC, Sullivan M, Du H, Feldman MI, Mendelson EB. "US Appearance of Ductal Carcinoma in situ." Radiographics. 2013 Jan-Feb;33(1):213-28. DOI: 10.1148/rg.331125092.
- Petrillo A, Fusco R, Petrillo M, Triunfo F, Filice S, Vallone P, Setola SV, Rubulotta M, Di Bonito M, Rinaldo M, D'Aiuto M, Brunetti A. "Added Value of Breast MRI for Preoperative Diagnosis of Ductal Carcinoma in situ: Diagnostic Performance on 362 Patients." Clin Breast Cancer. 2017 Jun;17(3):e127-e134. DOI: 10.1016/j.clbc.2016.12.007.
- Pinder SE. "Ductal Carcinoma in situ (DCIS): Pathological Features, Differential Diagnosis, Prognostic Factors and Specimen Evaluation." Mod Pathol. 2010 May;23 Suppl 2:S8-13. DOI: 10.1038/modpathol.2010.40.
- Quinn CM, Ostrowski JL. "Cytological and Architectural Heterogeneity in Ductal Carcinoma in situ of the Breast." J Clin Pathol. 1997 Jul;50(7):596-9. DOI: 10.1136/jcp.50.7.596.
- Faverly DR, Burgers L, Bult P, Holland R. "Three Dimensional Imaging of Mammary Ductal Carcinoma in situ: Clinical Implications." Semin Diagn Pathol. 1994 Aug;11(3):193-8. PMID: 7831530.
- Bellamy CO, McDonald C, Salter DM, Chetty U, Anderson TJ. "Noninvasive Ductal Carcinoma of the Breast: The Relevance of Histologic Categorization." Hum Pathol. 1993 Jan;24(1):16-23. DOI: 10.1016/0046-8177(93)90057-n.
- Groen EJ, Elshof LE, Visser LL, Rutgers EJT, Winter-Warnars HAO, Lips EH, Wesseling J. "Finding the Balance Between Over- and Under-treatment of Ductal Carcinoma in situ (DCIS)." Breast. 2017 Feb;31:274-283. DOI: 10.1016/j.breast.2016.09.001.
- Doke K, Butler S, Mitchell MP. "Current Therapeutic Approaches to DCIS." J Mammary Gland Biol Neoplasia. 2018 Dec;23(4):279-291. DOI: 10.1007/s10911-018-9415-1.